Method OIV-MA-AS315-18 Type II Method
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Safety Data Sheet
SAFETY DATA SHEET Revision date 02-Jun-2021 Revision Number 1 1. Identification Product identifier Product Name Fallow Star Other means of identification Product Code(s) 42750-63 UN/ID no. UN3082 Synonyms None Registration Number(s) 42750-63 Recommended use of the chemical and restrictions on use Recommended use Herbicide Restrictions on use Follow label instructions Details of the supplier of the safety data sheet Company Address ALBAUGH LLC 1525 NE 36th St, Ankeny, IA 50211 Emergency telephone number For Chemical Emergency Spill, Leak, Fire, Exposure, or Accident Call CHEMTREC Day or Night • Within USA and Canada: 1-800-424-9300 • Outside USA and Canada: +1 703-741-5970 (collect calls accepted) This product is an EPA FIFRA registered pesticide. Some classifications on this SDS are not the same as the FIFRA label. Certain sections of this SDS are superseded by federal law governed by US EPA for a registered pesticide. Please see Section 15. REGULATORY INFORMATION for explanation. 2. Hazard(s) identification Classification This chemical is not considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Hazards not otherwise classified (HNOC) Not applicable Label elements _____________________________________________________________________________________________ Page 1 / 10 42750 -63 - Fallow Star Revision date 02-Jun-2021 _____________________________________________________________________________________________ Hazard statements This chemical is not considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) The product contains no substances which at their given concentration, are considered to be hazardous to health. Appearance viscous Physical state Liquid Odor No data available Other information May be harmful in contact with skin. Toxic to aquatic life with long lasting effects. -

Toxicological Profile for Glyphosate Were
A f Toxicological Profile for Glyphosate August 2020 GLYPHOSATE II DISCLAIMER Use of trade names is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human Services. GLYPHOSATE III FOREWORD This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for these toxic substances described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced. The focus of the profiles is on health and toxicologic information; therefore, each toxicological profile begins with a relevance to public health discussion which would allow a public health professional to make a real-time determination of whether the presence of a particular substance in the environment poses a potential threat to human health. The adequacy of information to determine a substance's -
United States Patent to 11 4,011,057 Sayers 45) Mar
United States Patent to 11 4,011,057 Sayers 45) Mar. 8, 1977 54 HINDERED PHENOL ANTIOXIDANT 2,202,877 6, 1940 Stevens et al. ........................ 4.4/78 COMPOSTION CONTAINING AN AMNO 2,459,597 - 1/1949 Stillson et al...................... 44f78 X COMPOUND 2,521,425 9/1950 Thompson ............................. 44f75 . 2,672,408 3/1954 Bonner ........ a- - - - - - - a a a a - - 44f72 (75 Inventor: George Alvin Sayers, Penns Grove, 2,793,943 5/1957 Moore ...... a- a - a a - - - a a - a - 44f72 N.J. 2,908,558 10/1959 Brimer...... - - - - - - 44f75 73) Assignee: E. I. Du Pont de Nemours and 3,166,509 171965 Ecke ...................................... 44,178 Company, Wilmington, Del. Primary Examiner-Daniel E. Wyman 22 Filed: Apr. 16, 1974 Assistant Examiner-Mrs. Y. Harris-Smith (21) Appl. No.:& was 461,391 Attorney, Agent, or Firm-James A. Costello 52 U.S. Cl. ....................................... 44/52; 4.4/72; 57 ABSTRACT 51l Int. Cl”...........................................44/75; coL22 252/403 An improved antioxidant composition. comprising hin 58) Field of Search ........... 44/52, 72, 75; 252/403; dered phenol antioxidant wherein the improvement 260/624 comprises, in combination with the hindered phenol, 8 from about 1% to 10%, by total weight, of at least one 56 References Cited amino compound having a pKb of from 0.3 to 8.0. UNITED STATES PATENTS 1,748,507 111930 Brooks .................................. 44/52 12 Claims, No Drawings 401 1,057 1. 2 Ammonia has a pKa of 4.75 while substituted ammo HINDERED PHENOL ANTIOxIDANT nia compounds such as methylamine (pK 3.37), ethyl COMPOSTON CONTAINING AN AMNO amine (pK, 3.27), isopropylamine (pK 3.37), ethanol COMPOUND amine (pK 4.56) are slightly stronger bases than am monia. -

Supporting Information
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry C. This journal is © The Royal Society of Chemistry 2017 Supporting Information Colorimetric Sensor Array for Amines based on Responsive Lanthanide Complex Entrapment Peng Li, Zhiqiang Li, Decui Yao, and Huanrong Li* School of Chemical Engineering and Technology, Hebei University of Technology, Guang Rong Dao 8, Hongqiao District, Tianjin 300130, P. R. China. *E-mail: [email protected] 1 Figure S1 Molecular structure of HFA. Figure S2 XRD pattern of zeolite Y (ZY) Figure S3 SEM images of zeolite Y (ZY) 2 Figure S4 a) Emission spectra of Eu1Tb9(HFA)n@ZY after treatment with various amine vapors using an excitation wavelength of 302 nm. (Eu1Tb9(HFA)n@ZY (red line), aniline (green line), Benzylamine (blue line), Propylamine (cyan line), 1,3-Propanediamine (magenta line), Ethylenediamine (yellow line), Triethylamine (dark yellow line), Cyclohexylamine (navy line), Methylamine (purple line), N- Methylaniline (wine line), Butylamine (olive line), ammonia (dark cyan line), Tert-butylamine (royal line), Ethylamine (orange line)) b) The relative emission intensity at 612 nm and at 544 nm (IEu/ITb) of Eu1Tb9(HFA)n@ZY excitated at 302 nm upon treatment with various amine solvent vapors. (a: Eu1Tb9(HFA)n@ZY, b: Aniline, c: Benzylamine, d: Propylamine, e: 1,3-Propanediamine, f: Ethylenediamine, g: Triethylamine, h: Cyclohexylamine, i: Methylamine, j: N- Methylaniline , k: Butylamine, l: Ammonia, m: Tert-butylamine and n: Ethylamine) 3 Figure S5 a) Emission spectra of Eu0.5Tb9.5(HFA)n@ZY -

Poly(Vinylammonium Formate) and Process for Making Amidine
Europaisches Patentamt (19) European Patent Office Office europeenpeen des brevets EP0617 054B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) intci.e: C08F 8/12, C08F 8/44 of the grant of the patent: 04.12.1996 Bulletin 1996/49 (21) Application number: 94104538.7 (22) Date of filing: 22.03.1994 (54) Poly(vinylammonium formate) and process for making amidine-containing polymers Poly(Vinylammoniumformiat) und Verfahren zur Herstellung von Amidinegruppen enthaltender Polymeren Poly(formiate vinylique d'ammonium) et procede de preparation de polymeres contenant de groupes amidine (84) Designated Contracting States: (72) Inventor: Ford, Michael Edward BE DE FR GB NL Coopersburg, PA 18036 (US) (30) Priority: 25.03.1993 US 36757 (74) Representative: Kador & Partner Corneliusstrasse 15 (43) Date of publication of application: 80469 Miinchen (DE) 28.09.1994 Bulletin 1994/39 (56) References cited: (73) Proprietor: AIR PRODUCTS AND CHEMICALS, EP-A- 0 374 646 EP-A- 0 528 409 INC. DE-A-3 715 824 US-A- 4 393 174 Allentown, PA 18195-1501 (US) US-A- 4 943 676 DO ^> o Is- CO Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice the Patent Office of the Notice of shall be filed in o to European opposition to European patent granted. opposition a written reasoned statement. It shall not be deemed to have been filed until the opposition fee has been paid. (Art. a. 99(1) European Patent Convention). LU Printed by Jouve, 75001 PARIS (FR) EP 0 617 054 B1 Description FIELD OF THE INVENTION 5 This invention relates to poly(vinylammonium formate) and to a process for its manufacture. -

CHAPTER 6 Detection of Biogenic Amines: Quality and Toxicity Indicators in Food of Animal Origin
CHAPTER 6 Detection of Biogenic Amines: Quality and Toxicity Indicators in Food of Animal Origin César A. Lázaro de la Torre*,**, Carlos A. Conte-Junior**,† *National University of San Marcos, San Borja, Lima, Peru; **Federal Fluminense University, Niterói, Rio de Janeiro, Brazil; †Food Science Program, Chemistry Institute, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil 1 Biogenic Amines 1.1 Definition Biogenic amines (BAs) are low molecular weight substances, primarily produced during the normal metabolism of animals, plants, and microorganisms; their biological participation is related to psychoactive, neuroactive, or vasoactive process (Gloria, 2005; Ten Brink et al., 1990). Their presence in foods is directly related to microorganisms (spoilage bacterial) with decarboxylase activity on free amino acids (FAA). However, other factors, such as amino acid composition (kind of food), temperature, and time of storage, technological processes (maturation, packing, cooking, etc.) are important in BAs presentation (Halász et al., 1994). Most of the BAs have been named according to their amino acids precursors (histamine originates from histidine) and the most interesting classification is related to the number of amine groups: mono-, di-, and polyamines for tyramine, cadaverine, and spermidine respectively (Gloria, 2005). A graphic summary of BAs formation is presented in Fig. 6.1. 1.2 Classification Previously, we mentioned that BAs are classified based on the number of amine groups. However, chemical structure, biosynthesis, or physiological functions are used to divide them. Ruiz-Capillas and Jiménez-Colmenero (2004), Silla Santos (1996), and Shalaby (1996) presented a classification according to the number of amine groups: monoamines (tyramine, phenylethylamine), diamines (histamine, serotonin, tryptamine, putrescine, cadaverine), or polyamines (spermine, spermidine, agmatine). -

Evaluation of Manufacturing Processes for the Production of Atrazine
EVALUATION OF MANUFACTURING PROCESSES FOR THE PRODUCTION OF ATRAZINE by Melissa Claire Schaefer Baccalaureus Technologiae: Chemistry A dissertation submitted in fulfilment of the requirements for the Magister Technologiae : Chemistry In the Faculty of Applied Science at the PORT ELIZABETH TECHNIKON January 2002 Promoter: Prof. B. Zeelie View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by South East Academic Libraries System (SEALS) Look thoroughly inside yourself; there is a source of strength that will always issue forth should you seek it. ~ Marcus Aurelius ~ ACKNOWLEDGEMENTS • Professor Ben Zeelie for his input and valued advice. • Dr. Benita Barton for proofreading this dissertation. • Dr. Babrie Schoonees for her guidance. • Staff and students at the PE Technikon chemistry department. • My closest friend and confidant, Dr. Shawn Gouws for unconditional support and encouragement. • Mom, Dad and Selwyn, for endless love and motivation to strive for a dream. SUMMARY This report describes the results of investigations carried out with the view to find an alternative for MIBK as solvent for the production of atrazine as currently practised by Dow AgroSciences in South Africa. The main motivating factors for the said investigation was: • to increase the yield of atrazine produced, • to reduce the amount of organics, consisting essentially of reaction solvent containing dissolved product, in the aqueous process effluent, and • to improve the properties of the solid (crystalline) product to enable easier product formulation. Synthetic reactions carried out in the absence of organic solvent, i.e. in essentially a 10% NaCl solution containing a surfactant, proved rather disappointing. Low yields of atrazine were obtained together with relatively large amounts of by-products such as propazine and simazine, irrespective of the nature of the surfactant. -

Determination of Volatile Amines Using an Agilent J&W Select CP
Determination of Volatile Amines using an Agilent J&W Select CP-Volamine GC Column Application Note Introduction Volatile amines are among the most demanding analytes in gas chromatography due to their basic properties. The Select CP-Volamine WCOT fused silica column, coated with a base deactivated non-polar siloxane type stationary phase, is optimized for the separation of these amines. The application of proprietary base deactivation techniques creates a highly inert coating surface with a minimum degree of absorption for difficult basic compounds. The thick film siloxane type liquid phase coating layer offers optimal inertness as well as sufficient retention for the analysis of highly volatile and basic substances, such as methylamine and dimethylamine. The improved column inertness of the Select CP-Volamine column is evident from the good peak shape for these two difficult compounds in the chromatogram. The column also exhibits excellent stability for basic aqueous samples resulting in longer lifetimes even for these demanding samples. Figure 1 shows the separation of a complex mixture of amines in water, including the most volatile types. Conditions Table 1. Peak Identification Technique: GC-FID Peak RT (min) Name Column: Select CP-Volamine, 30 m x Peak RT (min) Name 0.32 mm 1 2,848 Methylamine 11 8,177 n-Butylamine (part number CP7447) 2 3,691 Dimethylamine 12 8,402 Ethylenediamine Sample Solvent: Water 3 3,904 Ethylamine previously 13 8,610 Ethanolamine Carrier Gas: Helium, 2.0 mL/min constant established retention time, 14 9,447 Triethylamine flow baseline separated from Injector: Split/splitless; Splitflow: 1:50 DMA and TMA). -
Safety Data Sheet
SAFETY DATA SHEET Revision date 23-Apr-2021 Revision Number 1 1. Identification Product identifier Product Name Aqua Star Other means of identification Product Code(s) 000065 Synonyms None Registration Number(s) 42750-059 Recommended use of the chemical and restrictions on use Recommended use Herbicide Restrictions on use Follow label instructions Details of the supplier of the safety data sheet Company Address ALBAUGH LLC 1525 NE 36th St, Ankeny, IA 50211 Emergency telephone number For Chemical Emergency Spill, Leak, Fire, Exposure, or Accident Call CHEMTREC Day or Night • Within USA and Canada: 1-800-424-9300 • Outside USA and Canada: +1 703-741-5970 (collect calls accepted) This product is an EPA FIFRA registered pesticide. Some classifications on this SDS are not the same as the FIFRA label. Certain sections of this SDS are superseded by federal law governed by US EPA for a registered pesticide. Please see Section 15. REGULATORY INFORMATION for explanation. 2. Hazard(s) identification Classification Acute toxicity - Inhalation (Dusts/Mists) Category 4 Serious eye damage/eye irritation Category 2B Hazards not otherwise classified (HNOC) Not applicable Label elements Warning _____________________________________________________________________________________________ Page 1 / 9 000065 - Aqua Star Revision date 23-Apr-2021 _____________________________________________________________________________________________ Hazard statements Harmful if inhaled Causes eye irritation Appearance viscous Physical state Liquid Odor Slight Organic Precautionary Statements - Prevention Avoid breathing dust/fume/gas/mist/vapors/spray Use only outdoors or in a well-ventilated area Wash face, hands and any exposed skin thoroughly after handling Precautionary Statements - Response IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. -

Department of Labor
Vol. 79 Friday, No. 197 October 10, 2014 Part II Department of Labor Occupational Safety and Health Administration 29 CFR Parts 1910, 1915, 1917, et al. Chemical Management and Permissible Exposure Limits (PELs); Proposed Rule VerDate Sep<11>2014 17:41 Oct 09, 2014 Jkt 235001 PO 00000 Frm 00001 Fmt 4717 Sfmt 4717 E:\FR\FM\10OCP2.SGM 10OCP2 mstockstill on DSK4VPTVN1PROD with PROPOSALS2 61384 Federal Register / Vol. 79, No. 197 / Friday, October 10, 2014 / Proposed Rules DEPARTMENT OF LABOR faxed to the OSHA Docket Office at Docket: To read or download (202) 693–1648. submissions or other material in the Occupational Safety and Health Mail, hand delivery, express mail, or docket go to: www.regulations.gov or the Administration messenger or courier service: Copies OSHA Docket Office at the address must be submitted in triplicate (3) to the above. All documents in the docket are 29 CFR Parts 1910, 1915, 1917, 1918, OSHA Docket Office, Docket No. listed in the index; however, some and 1926 OSHA–2012–0023, U.S. Department of information (e.g. copyrighted materials) Labor, Room N–2625, 200 Constitution is not publicly available to read or [Docket No. OSHA 2012–0023] Avenue NW., Washington, DC 20210. download through the Web site. All submissions, including copyrighted RIN 1218–AC74 Deliveries (hand, express mail, messenger, and courier service) are material, are available for inspection Chemical Management and accepted during the Department of and copying at the OSHA Docket Office. Permissible Exposure Limits (PELs) Labor and Docket Office’s normal FOR FURTHER INFORMATION CONTACT: business hours, 8:15 a.m. -
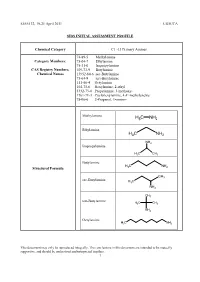
Sids Initial Assessment Profile
SIAM 32, 19-21 April 2011 US/ICCA SIDS INITIAL ASSESSMENT PROFILE Chemical Category C1 -13 Primary Amines 74-89-5 Methylamine Category Members: 75-04-7 Ethylamine 75-31-0 Isopropylamine CAS Registry Numbers, 109-73-9 Butylamine Chemical Names 13952-84-6 sec-Butylamine 75-64-9 tert-Butylamine 111-86-4 Octylamine 104-75-6 Hexylamine, 2-ethyl 5332-73-0 Propylamine, 3-methoxy- 1761-71-3 Cyclohexylamine, 4,4’-methylenebis 78-96-6 2-Propanol, 1-amino- Methylamine H C NH 3 2 Ethylamine H C NH 3 2 NH2 Isopropylamine H3C CH3 Butylamine H C NH Structural Formula 3 2 CH3 sec-Butylamine H3C NH 2 CH3 tert-Butylamine H3C CH3 NH2 Octylamine H C NH 3 2 This document may only be reproduced integrally. The conclusions in this document are intended to be mutually supportive, and should be understood and interpreted together. 1 SIAM 32, 19-21 April 2011 US/ICCA H3C NH2 Hexylamine, 2-ethyl H C 3 Propylamine, 3- O NH2 methoxy- H3C Cyclohexylamine, 4,4’-methylenebis H2N NH2 OH 2-Propanol, 1- amino- NH2 H3C SUMMARY CONCLUSIONS OF THE SIAR Category rationale The C10-C13 primary amines category is limited to the eleven sponsored substances as mentioned above. It excludes C12-C16 primary fat amines. The C10-C13 Primary Amines category is represented by the structure with a single and primary amino-group R- NH2, where R is an alkyl group that may be linear, branched or alicyclic; the alkyl group may include an atom or group that will not react with or substantially affect the properties of the amine function.