Groundwater Quality Assessment for Irrigation Use in Vadodara District, Gujarat, India S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter II: Study Area
Chapter II: Study Area CHAPTER II: STUDY AREA 2.0 Description of the Study area: Vadodara district is one of the most important districts of Gujarat. It is a leading agriculture district and one of the main contributors to the agricultural production in the state. 2.1 Geographical Location: Vadodara District is a district in the eastern part of the state of Gujarat in western India. It lies between latitudes 21° 45’ and 22° 45’ North and longitudes 72° 48’ and 74° 15’ East having a geographical area of 7,550 km². The district is bounded by Panchmahal and Dahod districts to the North, Anand and Kheda to the West, Bharuch and Narmada districts to the South, and the state of Madhya Pradesh to the East. Administratively, the district is subdivided into twelve talukas, viz. Vadodara, 28 Chapter II: Study Area Karjan, Padra, Savli, Dabhoi, Sankheda, Waghodia, Jetpur Pavi, Chhota Udepur, Naswadi, Tilakwada and Sinor. In the present work, part of Vadodara district is selected as a site of the study area which includes portions from Vadodara, Padra, Dabhoi and Waghodia talukas. Site of study area is shown in map given below (Figure 4). Fig 4. Map showing site of study area The Mahi River passes through the district. Orsang, Dhadhar, Dev, Goma, Jambuva, Vishwamitri, Bhukhi Heran, Mesari, Karad, Men, Ani, Aswini and Sukhi are the small rivers. Minor irrigation dams are constructed across Sukhi and Rami rivers. Geographically, the district comprises of Khambhat Silt in the south-west, Mahi plain in the north-west, Vadodara plain in the middle, Orsang-Heran plain in the mid-east, Vindhyan hills in the east and Narmada gorge in the south-east which merges westwards 29 Chapter II: Study Area with the lower Narmada Valley. -

Junagadh Agricultural University Junagadh-362 001
Junagadh Agricultural University Junagadh-362 001 Information Regarding Registered Students in the Junagadh Agricultural University, Junagadh Registered Sr. Name of the Major Minor Remarks Faculty Subject for the Approved Research Title No. students Advisor Advisor (If any) Degree 1 Agriculture Agronomy M.A. Shekh Ph.D. Dr. M.M. Dr. J. D. Response of castor var. GCH 4 to irrigation 2004 Modhwadia Gundaliya scheduling based on IW/CPE ratio under varying levels of biofertilizers, N and P 2 Agriculture Agronomy R.K. Mathukia Ph.D. Dr. V.D. Dr. P. J. Response of castor to moisture conservation 2005 Khanpara Marsonia practices and zinc fertilization under rainfed condition 3 Agriculture Agronomy P.M. Vaghasia Ph.D. Dr. V.D. Dr. B. A. Response of groundnut to moisture conservation 2005 Khanpara Golakia practices and sulphur nutrition under rainfed condition 4 Agriculture Agronomy N.M. Dadhania Ph.D. Dr. B.B. Dr. P. J. Response of multicut forage sorghum [Sorghum 2006 Kaneria Marsonia bicolour (L.) Moench] to varying levels of organic manure, nitrogen and bio-fertilizers 5 Agriculture Agronomy V.B. Ramani Ph.D. Dr. K.V. Dr. N.M. Efficiency of herbicides in wheat (Triticum 2006 Jadav Zalawadia aestivum L.) and assessment of their persistence through bio assay technique 6 Agriculture Agronomy G.S. Vala Ph.D. Dr. V.D. Dr. B. A. Efficiency of various herbicides and 2006 Khanpara Golakia determination of their persistence through bioassay technique for summer groundnut (Arachis hypogaea L.) 7 Agriculture Agronomy B.M. Patolia Ph.D. Dr. V.D. Dr. B. A. Response of pigeon pea (Cajanus cajan L.) to 2006 Khanpara Golakia moisture conservation practices and zinc fertilization 8 Agriculture Agronomy N.U. -

(PANCHAYAT) Government of Gujarat
ROADS AND BUILDINGS DEPARTMENT (PANCHAYAT) Government of Gujarat ENVIRONMENTAL AND SOCIAL IMPACT ASSESSMENT (ESIA) FOR GUJARAT RURAL ROADS (MMGSY) PROJECT Under AIIB Loan Assistance May 2017 LEA Associates South Asia Pvt. Ltd., India Roads & Buildings Department (Panchayat), Environmental and Social Impact Government of Gujarat Assessment (ESIA) Report Table of Content 1 INTRODUCTION ............................................................................................................. 1 1.1 BACKGROUND .......................................................................................................... 1 1.2 MUKHYA MANTRI GRAM SADAK YOJANA ................................................................ 1 1.3 SOCIO-CULTURAL AND ECONOMIC ENVIRONMENT: GUJARAT .................................... 3 1.3.1 Population Profile ........................................................................................ 5 1.3.2 Social Characteristics ................................................................................... 5 1.3.3 Distribution of Scheduled Caste and Scheduled Tribe Population ................. 5 1.3.4 Notified Tribes in Gujarat ............................................................................ 5 1.3.5 Primitive Tribal Groups ............................................................................... 6 1.3.6 Agriculture Base .......................................................................................... 6 1.3.7 Land use Pattern in Gujarat ......................................................................... -

Madhya Gujarat Vij Company Limited Name Designation Department Email-Id Contact No Mr
Madhya Gujarat Vij Company Limited Name Designation Department email-id Contact No Mr. Rajesh Manjhu,IAS Managing Director Corporate Office [email protected] 0265-2356824 Mr. K R Shah Sr. Chief General Manager Corporate Office [email protected] 9879200651 Mr. THAKORPRASAD CHANDULAL CHOKSHI Chief Engineer Corporate Office [email protected] 9879202415 Mr. K N Parikh Chief Engineer Corporate Office [email protected] 9879200737 Mr. Mayank G Pandya General Manager Corporate Office [email protected] 9879200689 Mr. KETAN M ANTANI Company Secretary Corporate Office [email protected] 9879200693 Mr. H R Shah Additional Chief Engineer Corporate Office [email protected] 9925208253 Mr. M T Sanghada Additional Chief Engineer Corporate Office [email protected] 9925208277 Mr. P R RANPARA Additional General Manager Corporate Office [email protected] 9825083901 Mr. V B Gandhi Additional Chief Engineer Corporate Office [email protected] 9925208141 Mr. BHARAT J UPADHYAY Additional Chief Engineer Corporate Office [email protected] 9925208224 Mr. S J Shukla Superintending Engineer Corporate Office [email protected] 9879200911 Mr. M M Acharya Superintending Engineer Corporate Office [email protected] 9925208282 Mr. Chandrakant N Pendor Superintending Engineer Corporate Office [email protected] 9925208799 Mr. Jatin Jayantilal Parikh Superintending Engineer Corporate Office [email protected] 9879200639 Mr. BIHAG C MAJMUDAR Superintending Engineer Corporate Office [email protected] 9925209512 Mr. Paresh Narendraray Shah Chief Finance Manager Corporate Office [email protected] 9825603164 Mr. Harsad Maganbhai Patel Controller of Accounts Corporate Office [email protected] 9925208189 Mr. H. I. PATEL Deputy General Manager Corporate Office [email protected] 9879200749 Mr. -

Sub-Basalt Imaging in Padra Field, South Cambay Basin, India Through Sub-Surface Angle Domain Processing Abhinandan Ghosh1*, Debkumar Chatterjee1 and C.P.S
Sub-basalt imaging in Padra Field, South Cambay Basin, India through sub-surface angle domain processing Abhinandan Ghosh1*, Debkumar Chatterjee1 and C.P.S. Rana1 1SPIC, GPS, WOB, ONGC, Mumbai *[email protected] Abstract Penetration of seismic energy below basalt and generation of sub-basalt imaging has always been a challenging task for seismic exploration. Strong inter-bed multiples always mask the weak reflections coming from sub-basalt formations. Severe scattering of seismic energy due to heterogeneity of basalt layer further complicates the problem which results in the lowering of frequency content of the data and also velocity model building for imaging becomes highly challenging. Padra Field in South Cambay Basin, India is no different, where basaltic Deccan Trap is the technical basement. Conventional PSTM vintages of this area imaged shallow sedimentary part only without much success in imaging the sub-basalt part. These challenges have been addressed and successful sub-basalt imaging is being achieved through full azimuth sub- surface angle domain processing. In this paper we describe a workflow designed to better image the basement, and fine scale features within the basement, at Padra Field. Introduction Sub-basalt imaging continues to provide a challenge in the Padra area. The ability to image seismically the subsurface below the basalts is limited by the fact that basaltic layers are characterized by the poor penetration because of high reflectivity contrasts at the top of the basalt and also due to intra basalt discontinuities, attenuation and strong internal scattering of seismic energy due to heterogeneity of basalt layer resulting lowering of the frequency content of the seismic data. -

Gujarat Cotton Crop Estimate 2019 - 2020
GUJARAT COTTON CROP ESTIMATE 2019 - 2020 GUJARAT - COTTON AREA PRODUCTION YIELD 2018 - 2019 2019-2020 Area in Yield per Yield Crop in 170 Area in lakh Crop in 170 Kgs Zone lakh hectare in Kg/Ha Kgs Bales hectare Bales hectare kgs Kutch 0.563 825.00 2,73,221 0.605 1008.21 3,58,804 Saurashtra 19.298 447.88 50,84,224 18.890 703.55 78,17,700 North Gujarat 3.768 575.84 12,76,340 3.538 429.20 8,93,249 Main Line 3.492 749.92 15,40,429 3.651 756.43 16,24,549 Total 27.121 512.38 81,74,214 26.684 681.32 1,06,94,302 Note: Average GOT (Lint outturn) is taken as 34% Changes from Previous Year ZONE Area Yield Crop Lakh Hectare % Kgs/Ha % 170 kg Bales % Kutch 0.042 7.46% 183.21 22.21% 85,583 31.32% Saurashtra -0.408 -2.11% 255.67 57.08% 27,33,476 53.76% North Gujarat -0.23 -6.10% -146.64 -25.47% -3,83,091 -30.01% Main Line 0.159 4.55% 6.51 0.87% 84,120 5.46% Total -0.437 -1.61% 168.94 32.97% 25,20,088 30.83% Gujarat cotton crop yield is expected to rise by 32.97% and crop is expected to increase by 30.83% Inspite of excess and untimely rains at many places,Gujarat is poised to produce a very large cotton crop SAURASHTRA Area in Yield Crop in District Hectare Kapas 170 Kgs Bales Lint Kg/Ha Maund/Bigha Surendranagar 3,55,100 546.312 13.00 11,41,149 Rajkot 2,64,400 714.408 17.00 11,11,115 Jamnagar 1,66,500 756.432 18.00 7,40,858 Porbandar 9,400 756.432 18.00 41,826 Junagadh 74,900 756.432 18.00 3,33,275 Amreli 4,02,900 756.432 18.00 17,92,744 Bhavnagar 2,37,800 756.432 18.00 10,58,115 Morbi 1,86,200 630.360 15.00 6,90,430 Botad 1,63,900 798.456 19.00 7,69,806 Gir Somnath 17,100 924.528 22.00 92,997 Devbhumi Dwarka 10,800 714.408 17.00 45,386 TOTAL 18,89,000 703.552 16.74 78,17,700 1 Bigha = 16 Guntha, 1 Hectare= 6.18 Bigha, 1 Maund= 20 Kg Saurashtra sowing area reduced by 2.11%, estimated yield increase 57.08%, estimated Crop increase by 53.76%. -

Disaster Risk Management Programme at the Community Level
Evaluation Team Santosh Kumar K. Mukundan Nisheeth Kumar Prema Gopalan Kenneth Westgate EXECUTIVE SUMMARY Overall Assessment 1. Ministry of Home Affairs, Government of India had launched first of its kind a disaster risk management Programme at the community level. The Programme was supported by UNDP. The main objective of the programme was to reduce vulnerability of the community by building local capacity. The purpose of this evaluation exercise is to carry out a rapid qualitative assessment of the Phase 1 of Government of India-UNPD Disaster Risk Management (DRM) Program implemented across Bihar, Orissa and Gujarat. The evaluation has twin objectives of learning and application of learning to improve the program performance. 2. Overall the program has been in a position to advance the disaster mitigation agenda and most actions in this regard are focused on preparedness for better response. There is a shift in understanding that natural disasters are not the wrath of God and that this can be fought with pre planning initiatives. The strength of the program design is the ability of partners to innovate as part of implementation for this happen. There is a need for stronger strategic management of the program. So as to integrate it with state governments efforts for disaster risk management at community level. 3. The stated design of the project has been ambitious in view of the available resources and time available for its implementation. Given the resources (US$ 34 million), the question that is constantly raised is as to whether the program is spread too thinly over larger number of districts with too many components. -
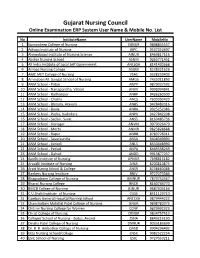
Gujarat Nursing Council Online Examination ERP System User Name & Mobile No
Gujarat Nursing Council Online Examination ERP System User Name & Mobile No. List No InstituteName UserName MobileNo 1 Sumandeep College of Nursing SUNUR 9898855557 2 Adivasi Institute of Nursing AIPC 9537352497 3 Ahmedabad Institute of Nursing Science AINUR 8469817116 4 Akshar Nursing School ASNM 9265771451 5 All India Institute of Local Self Government ANLSGA 8141430568 6 Ambaji Nursing College ASGM 8238321626 7 AMC MET College of Nursing VSAS 9328259403 8 Aminaben M. Gangat School of Nursing AMGS 7435011893 9 ANM School - Patan ANPP 9879037592 10 ANM School - Nanapondha, Valsad ANVV 9998994841 11 ANM School - Radhanpur ANRP 9426260500 12 ANM School - Chotila ANCS 7600050420 13 ANM School - Bhiloda, Aravalli ANBS 9428482016 14 ANM School - Bavla ANBA 9925252386 15 ANM School - Padra, Vadodara ANPV 9427842208 16 ANM School - Sachin, Surat ANSS 8160485736 17 ANM School - Visnagar ANVM 9979326479 18 ANM School - Morbi ANMR 9825828688 19 ANM School - Rapar ANRB 8780726011 20 ANM School - Savarkundla ANSA 9408349990 21 ANM School - Limbdi ANLS 8530448990 22 ANM School - Petlad ANPA 8469538269 23 ANM School - Dahod ANDD 9913877237 24 Apollo Institute of Nursing APNUR 7698815182 25 Aravalli Institute of Nursing AINA 8200810875 26 Arpit Nuring School & College ANSR 8238660088 27 Bankers Nursing Institute BNIV 9727073584 28 Bhagyalaxmi College of Nursing BMNUR 7874752567 29 Bharat Nursing College BNCR 8160744770 30 BMCB College of Nursing BJNUR 9687404164 31 C.U.Shah Institute of Nursing CUSS 8511123710 32 Cambay General Hospital Nursing School ANTCKA 9879444223 33 Chanchalben Mafatlal Patel College of Nursing GNUR 9898780375 34 Chitrini Nursing College for Women CCNP 9829992323 35 Christ College of Nursing CRNUR 9834757510 36 College/ School of Nursing - Zydus, Anand ZSNA 8849216190 37 Dinsha Patel College of Nursing DNNUR 9033183699 38 Dr. -

Chapter: Ii Regional Setup: Physical and Socio-Economic
CHAPTER: II REGIONAL SETUP: PHYSICAL AND SOCIO-ECONOMIC 2.1 Introduction Vadodara district with 7548.50 Sq km area, is located central part of mainland Gujarat, lies between 21°49‟19” and 22°48‟37” north latitude and 72°51‟05”and 74°16‟55” east longitude. It falls in the Survey of India, degree sheets numbered 46B, 46F, 46J & 46G. The district is bounded in north & northeast by Anand, Panchmahals & Dahod districts, in east & in south east by Madhya Pardesh & Maharashtra State, in south east by Narmada district & in south & in west by Bharuch district. Vadodara city, the district headquarter is about 100 km south of Ahmedabad, is well connected to other parts of the State & Country by network of highways and railway network. Vadodara is redesigned in 1997 on the grounds that entire Tilakwada Taluka and a few towns of Nasvadi Taluka of the district has been moved in recently made Narmada region. Kavant Taluka has been recently made from Chhota Udaipur Taluka of the district. In this way at present the district has 12 talukas with 1,553 villages and 16 towns. Figure 2.1 Taluka Boundaries and Taluka Headquarters of Vadodara District. (Source: CGWB Report) Figure 2.1 delineates Administrative boundaries of Vadodara area alongside district head quarter and taluka limits with taluka head office, Vadodara region is subdivided into twelve 21 Talukas, viz. Vadodara, Savli, Vaghoriya, Sankheda, Jetpur Pavi, Chota Udaipur, Kawant, Naswadi, Dabhol, Sinor, Karjan and Padra. 2.2 Physical Aspect 2.2.1 Physiography: Vadodara region shapes a piece of the great Gujarat plain. -

Sl. No Name of the Beneficiary Parent Name Age Gender Caste Address 1 Megh Patel Hitesh Kumar 10Yrs Male G
Sl. No Name of the Beneficiary Parent Name Age Gender Caste Address 1 Megh Patel Hitesh Kumar 10Yrs Male G. Parnashil Residency Bwehind Krishna Park Ajwa Waghod Ring Road, Vadodara. 2 Mital Ben Vinod Bhai 11Yrs Female 69-Janka Nagar, Society Mothers School Road, Near Jailar Malenagar. 3 Nisargohil Alpesh Singh 7Yrs Male C-15, Kiritmandir, Staff Quarters, Near Aaradana Cinema, Saltwada, Vadodara. 4 Manav Patel Vasanth Bhai 11Yrs Male Sri Malenagar, Ambika Nagar, Pachal Svvast, Vododara 5 Devparte Dinesh Bhai 7Yrs Male 1-Tej Quarters Behind Urmi Apartment, Fateachgunj, Vadodara 6 Deepiika Pagare Kishore 7Yrs Female Gokul Nagar, Gotri Road. 7 Vrushika Patel Vishnu Bhai 10 Yrs Female Parot Faliyu-1Vadsar, Gam, Vadodara Mandal 8 Faiza Patel Ismail 9Yrs Female 3-17, Madura Ramalesociety Near Jp Poloce Station, Tandaza. 9 Priyansh Patel Mayanek Patel 10 Yrs Male A-7-Shanti Kunj Soc Opp Raj Nagar Arunachal Samia Road, Vadodara 10 Dakshparekam Umesh Bhai 10Yrs Male Plot-83, Eev Nagar, 2 Old Pared Road, Biwualipura 11 Rana Harsh Kiran Kumar 11Yrs Male C-21, Saurabhtenament,Nrch Vidiyilaya 12 Nishth Shah Arvinod Bhai 25Yrs Male 27, Divyak Society, Mala Pur Vadodara. 13 Ritesh Parmar Arvinod Bhai 22Yrs Male Mu. Po. Vadodara Somnaith Namasaosu Vadodara 14 Bipin Garasiya Ramesh Bhai 25 Yrs Male Vidtiyash Nagar Colony Old Ladra Nagar Vadodara 15 Vaibhav Kapsi Girish Bhai 22 Yrs Male 148, Sgavati Nagar Near Mugger School , Vadodara 16 Vaibhav Kapsi Girish Bhai 22 Yrs Male 148, Sgavati Nagar Near Mugger School , Vadodara 17 Anil Panchal Jayanti 22 Yrs Male 1350 Ambika Nagar,Gotri Road Vadodara. -

Gujarat State
CENTRAL GROUND WATER BOARD MINISTRY OF WATER RESOURCES, RIVER DEVELOPMENT AND GANGA REJUVENEATION GOVERNMENT OF INDIA GROUNDWATER YEAR BOOK – 2018 - 19 GUJARAT STATE REGIONAL OFFICE DATA CENTRE CENTRAL GROUND WATER BOARD WEST CENTRAL REGION AHMEDABAD May - 2020 CENTRAL GROUND WATER BOARD MINISTRY OF WATER RESOURCES, RIVER DEVELOPMENT AND GANGA REJUVENEATION GOVERNMENT OF INDIA GROUNDWATER YEAR BOOK – 2018 -19 GUJARAT STATE Compiled by Dr.K.M.Nayak Astt Hydrogeologist REGIONAL OFFICE DATA CENTRE CENTRAL GROUND WATER BOARD WEST CENTRAL REGION AHMEDABAD May - 2020 i FOREWORD Central Ground Water Board, West Central Region, has been issuing Ground Water Year Book annually for Gujarat state by compiling the hydrogeological, hydrochemical and groundwater level data collected from the Groundwater Monitoring Wells established by the Board in Gujarat State. Monitoring of groundwater level and chemical quality furnish valuable information on the ground water regime characteristics of the different hydrogeological units moreover, analysis of these valuable data collected from existing observation wells during May, August, November and January in each ground water year (June to May) indicate the pattern of ground water movement, changes in recharge-discharge relationship, behavior of water level and qualitative & quantitative changes of ground water regime in time and space. It also helps in identifying and delineating areas prone to decline of water table and piezometric surface due to large scale withdrawal of ground water for industrial, agricultural and urban water supply requirement. Further water logging prone areas can also be identified with historical water level data analysis. This year book contains the data and analysis of ground water regime monitoring for the year 2018-19. -

List of State Office Bearers- Gujarat
List of State Office Bearers- Gujarat Chief Organiser 1 Shri Mangal Singh Solanki, Advocate Chief Organiser Gujarat Pradesh Congress Seva Dal 31ST Bhagatjalram Society Near Vejalpur Nagar Palika Vejalpur, Ahmedabad-51 Gujarat Tel: 079- 26810795, 098240-40196 26814873, Mahila Organiser 1 Ms. Minal Bhailalbhai Gohil Mahila Organiser Gujarat Pradesh Congress Seva Dal Virasa's Pole Opp. Giriraj Medical Store Ghadualia Pole, M.G. Road Vadodara- 390001 Gujarat Tel: 09427349699 Organisers 1. Shri Kumpaji Zala Organiser Gujarat Pradesh Congress Seva Dal Rampura, PO-Chaveli Tehsil-Chanasma, Via-Dhinoj Distt-Patan Gujarat Tel-02734-288558, 09825874853 Organising Secretaries 1 Shri Moti Lakhan 2 Sh Chandulbhai Damjibhai Badrukiya Organising Secretary Organising Secretary Gujarat Pradesh Congress Seva Dal Gujarat Pradesh Congress Seva Dal Jethalal Chali, B/s Vishal Medical Opp. Raiya Panchayat Office Behrampura Raiya Ahmedabad Dist- Rajkot Gujarat Gujarat 09825493892 Tel: 2581888, 9825218605 3 Shri Ishwarbhai Parmar 4 Sri Kamalsinh Rana, Advocate Organising Secretary Organising Secretary Gujarat Pradesh Congress Seva Dal Gujarat Pradesh Congress Seva Dal B/12, Pankaj Society Navdurga, 26, Saujanya Society Chakalia Road, Dahod Near Sargam Apartment Gujarat B/h Anandnagar, Karelibag Tel-09428128149 Baroda-18 Gujarat 5 Shri Prakashchandra Bharatiya 6 Shri Bipinchandra Rathod, Advocate Organising Secretary Organising Secretary Gujarat Pradesh Congress Seva Dal Gujarat Pradesh Congress Seva Dal 5, Chandralok Society Thakkarbapa Vaas At/PO-Deesa, B/H Laxmi