Federal Register/Vol. 86, No. 91/Thursday, May 13, 2021/Rules
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2020-2021 School Calendar Revised May 12, 2021
2020-2021 School Calendar Revised May 12, 2021 JULY 2020 First Day of School JANUARY 2021 Sun Mon Tues Wed Thurs Fri Sat Sun Mon Tues Wed Thurs Fri Sat Sept. 8 1 2 3 4 1 2 5 6 7 8 9 10 11 Holidays 3 4 5 6 7 8 9 Sept. 7 - Labor Day 12 13 14 15 16 17 18 10 11 12 13 14 15 16 Nov. 3 - Election Day 19 20 21 22 23 24 25 Nov. 11 - Veterans Day 17 18 19 20 21 22 23 26 27 28 29 30 31 Nov. 26 & 27 - Thanksgiving 24 25 26 27 28 29 30 Dec. 23, 2020–Jan. 1, 2021 - Winter Break 31 AUGUST 2020 Jan. 18 - Martin Luther King Jr. Day FEBRUARY 2021 Sun Mon Tues Wed Thurs Fri Sat Feb. 15 - Presidents Day Sun Mon Tues Wed Thurs Fri Sat 1 April 5-9 - Spring Break 1 2 3 4 5 6 May 31 - Memorial Day 2 3 4 5 6 7 8 7 8 9 10 11 12 13 9 10 11 12 13 14 15 Staff Days (no school for students) 14 15 16 17 18 19 20 Aug. 28, 31 Feb. 1 16 17 18 19 20 21 22 Sept. 2–4 March 5* 21 22 23 24 25 26 27 23 24 25 26 27 28 29 *only to be used if students are receiving 28 30 31 in-school instruction 5 days/week SEPTEMBER 2020 MARCH 2021 Sun Mon Tues Wed Thurs Fri Sat Flexible Staff Day Sun Mon Tues Wed Thurs Fri Sat August 27 June 21 1 2 3 4 5 1 2 3 4 5 6 6 7 8 9 10 11 12 Adjusted Dismissal for All Students 7 8 9 10 11 12 13 13 14 15 16 17 18 19 Nov. -

2021-2022 Custom & Standard Information Due Dates
2021-2022 CUSTOM & STANDARD INFORMATION DUE DATES Desired Cover All Desired Cover All Delivery Date Info. Due Text Due Delivery Date Info. Due Text Due May 31 No Deliveries No Deliveries July 19 April 12 May 10 June 1 February 23 March 23 July 20 April 13 May 11 June 2 February 24 March 24 July 21 April 14 May 12 June 3 February 25 March 25 July 22 April 15 May 13 June 4 February 26 March 26 July 23 April 16 May 14 June 7 March 1 March 29 July 26 April 19 May 17 June 8 March 2 March 30 July 27 April 20 May 18 June 9 March 3 March 31 July 28 April 21 May 19 June 10 March 4 April 1 July 29 April 22 May 20 June 11 March 5 April 2 July 30 April 23 May 21 June 14 March 8 April 5 August 2 April 26 May 24 June 15 March 9 April 6 August 3 April 27 May 25 June 16 March 10 April 7 August 4 April 28 May 26 June 17 March 11 April 8 August 5 April 29 May 27 June 18 March 12 April 9 August 6 April 30 May 28 June 21 March 15 April 12 August 9 May 3 May 28 June 22 March 16 April 13 August 10 May 4 June 1 June 23 March 17 April 14 August 11 May 5 June 2 June 24 March 18 April 15 August 12 May 6 June 3 June 25 March 19 April 16 August 13 May 7 June 4 June 28 March 22 April 19 August 16 May 10 June 7 June 29 March 23 April 20 August 17 May 11 June 8 June 30 March 24 April 21 August 18 May 12 June 9 July 1 March 25 April 22 August 19 May 13 June 10 July 2 March 26 April 23 August 20 May 14 June 11 July 5 March 29 April 26 August 23 May 17 June 14 July 6 March 30 April 27 August 24 May 18 June 15 July 7 March 31 April 28 August 25 May 19 June 16 July 8 April 1 April 29 August 26 May 20 June 17 July 9 April 2 April 30 August 27 May 21 June 18 July 12 April 5 May 3 August 30 May 24 June 21 July 13 April 6 May 4 August 31 May 25 June 22 July 14 April 7 May 5 September 1 May 26 June 23 July 15 April 8 May 6 September 2 May 27 June 24 July 16 April 9 May 7 September 3 May 28 June 25. -

2020-2021 Academic Year Grid ALL 11X17
Fall 2020 Spring 2021 Summer 2021* EVENTS / DEADLINES Session 1 Session 1 Session 2 Session 3 Session 4 Session 5 Session 6 Winter Mini Session 2 Session 3 Session 4 Session 5 Session 6 Summer Mini Session 1 Session 2 Session 3 Session 4 Regular Regular First Day of Classes September 28, October 19, November 2, December 21, February 22, *Please also see notes below August 24, 2020 August 24, 2020 August 24, 2020 January 19, 2021 January 19, 2021 January 19, 2021 March 22, 2021 April 5, 2021 May 17, 2021 June 7, 2021 June 7, 2021 June 7, 2021 July 12, 2021 2020 2020 2020 2020 2021 regarding college-specific dates and Monday Monday Monday Tuesday Tuesday Tuesday Monday Monday Monday Monday Monday Monday Monday Monday Monday Monday Monday Monday summer sessions meeting days. Labor Day Holiday (Fall); September 7, 2020 January 18, 2021 May 31, 2021 Martin Luther King Holiday (Spring); Monday Monday Monday Memorial Day (Summer) **Extended** September 30, October 21, November 4, December 22, February 24, Last Day to Add a Class September 1, August 26, 2020 August 26, 2020 January 26, 2021 January 21, 2021 January 21, 2021 March 24, 2021 April 7, 2021 May 18, 2021 June 8, 2021 June 8, 2021 June 8, 2021 July 13, 2021 2020 2020 2020 2020 2021 or be enrolled from the Wait List 2020 Wednesday Wednesday Tuesday Thursday Thursday Wednesday Wednesday Tuesday Tuesday Tuesday Tuesday Tuesday Wednesday Wednesday Wednesday Tuesday Wednesday Tuesday ORD - Official Reporting Day Last day to drop a course or withdraw without receiving a grade Last day to -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -

Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 3, 2021 ↔ November 4, 2020 January 4, 2021 ↔ November 5, 2020 January 5, 2021 ↔ November 6, 2020 January 6, 2021 ↔ November 7, 2020 January 7, 2021 ↔ November 8, 2020 January 8, 2021 ↔ November 9, 2020 January 9, 2021 ↔ November 10, 2020 January 10, 2021 ↔ November 11, 2020 January 11, 2021 ↔ November 12, 2020 January 12, 2021 ↔ November 13, 2020 January 13, 2021 ↔ November 14, 2020 January 14, 2021 ↔ November 15, 2020 January 15, 2021 ↔ November 16, 2020 January 16, 2021 ↔ November 17, 2020 January 17, 2021 ↔ November 18, 2020 January 18, 2021 ↔ November 19, 2020 January 19, 2021 ↔ November 20, 2020 January 20, 2021 ↔ November 21, 2020 January 21, 2021 ↔ November 22, 2020 January 22, 2021 ↔ November 23, 2020 January 23, 2021 ↔ November 24, 2020 January 24, 2021 ↔ November 25, 2020 January 25, 2021 ↔ November 26, 2020 January 26, 2021 ↔ November 27, 2020 January 27, 2021 ↔ November 28, 2020 January 28, 2021 ↔ November 29, 2020 January 29, 2021 ↔ November 30, 2020 January 30, 2021 ↔ December 1, 2020 January 31, 2021 ↔ December 2, 2020 February 1, 2021 ↔ December 3, 2020 February 2, 2021 ↔ December 4, 2020 1st Day 1st Day of Your Desired Stay you may Call February 3, 2021 ↔ December 5, 2020 February 4, 2021 ↔ December 6, 2020 February 5, 2021 ↔ December 7, 2020 February 6, 2021 ↔ December 8, 2020 February 7, 2021 ↔ December 9, 2020 February 8, 2021 ↔ December 10, 2020 February 9, 2021 ↔ December 11, 2020 February 10, 2021 ↔ December 12, 2020 February 11, 2021 ↔ December 13, 2020 -
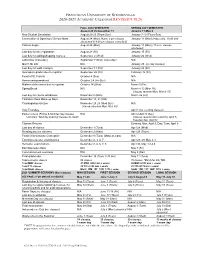
2020-2021 Academic Calendar Revised 9.18.20
FRANCISCAN UNIVERSITY OF STEUBENVILLE 2020-2021 ACADEMIC CALENDAR REVISED 9.18.20 FALL 2020 SEMESTER SPRING 2021 SEMESTER August 24 25-December 11 January 11-May 5 New Student Orientation August 20-23 (Thurs-Sun) January 7-10 (Thurs-Sun) Convocation & Opening of School Mass August 24 (Mon) (4 pm; 3 pm classes January 11 (Mon) (mass only, 10:30 am) shortened & 4:30 pm classes cancelled) Classes begin August 24 (Mon) January 11 (Mon) (10 a.m. classes shortened) Last day for late registration August 28 (Fri) January 15 (Fri) Last day for adding/dropping courses September 2 (Wed) January 20 (Wed) Labor Day (class day) September 7 (Mon) (class day) N/A March for Life N/A January 29 (no day classes) Last day for audit changes September 11 (Fri) January 22 (Fri) Incomplete grades due to registrar September 25 (Fri) February 12 (Fri) Feast of St. Francis October 4 (Sun) N/A Homecoming weekend October 2-4 (Fri-Sun) N/A Midterm deficiencies due to registrar October 14 (Wed) March 5 (Fri) Spring Break N/A March 8-12 (Mon-Fri) (classes resume Mon, March 15) Last day for course withdrawal November 2 (Mon) March 26 (Fri) Tentative Class Make-up Days November 14, 21 (Sat) Thanksgiving vacation November 25-29 (Wed-Sun) N/A (classes resume Mon, Nov 30) Holy Thursday April 1 (no evening classes) Easter recess (Friday & Monday day classes N/A April 2-April 5 (day) canceled; *Monday evening classes do meet) (classes resume Mon evening, April 5, Tuesday day, April 6) Classes Resume Evening: Mon, April 5; Day: Tues, April 6 Last day of classes December 1 (Tues) -
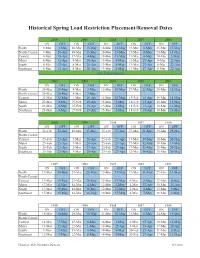
Historical Spring Load Restriction Placement/Removal Dates
Historical Spring Load Restriction Placement/Removal Dates 2010 20092008 2007 2006 ON OFF ON OFF ON OFF ON OFF ON OFF North 9-Mar 3-May 18-Mar 13-May 18-Mar 13-May 13-Mar 8-May 25-Mar 15-May North-Central 9-Mar 26-Apr 18-Mar 13-May 18-Mar 13-May 13-Mar 8-May 24-Mar 15-May Central 9-Mar 19-Apr 17-Mar 4-May 18-Mar 13-May 11-Mar 4-May 10-Mar 5-May Metro 8-Mar 12-Apr 8-Mar 20-Apr 14-Mar 9-May 11-Mar 27-Apr 8-Mar 22-Apr South 8-Mar 12-Apr 8-Mar 20-Apr 13-Mar 8-May 11-Mar 27-Apr 8-Mar 22-Apr Southeast 8-Mar 12-Apr 8-Mar 20-Apr 13-Mar 8-May 11-Mar 27-Apr 8-Mar 22-Apr New Policy Enacted 2005 2004 2003 2002 2001 ON OFF ON OFF ON OFF ON OFF ON OFF North 26-Mar 20-May 8-Mar 3-May 15-Mar 10-May 27-Mar 22-May 20-Mar 15-May North-Central 26-Mar 20-May 8-Mar 3-May Central 25-Mar 12-May 1-Mar 26-Apr 15-Mar 10-May 19-Feb 16-Apr 19-Mar 14-May Metro 23-Mar 4-May 27-Feb 19-Apr 15-Mar 2-May 18-Feb 15-Apr 18-Mar 13-May South 23-Mar 4-May 27-Feb 19-Apr 15-Mar 2-May 18-Feb 15-Apr 18-Mar 13-May Southeast 23-Mar 4-May 27-Feb 19-Apr 15-Mar 2-May 18-Feb 15-Apr 18-Mar 13-May 2000 1999 1998 1997 1996 ON OFF ON OFF ON OFF ON OFF ON OFF North 26-Feb 22-Apr 18-Mar 13-May 25-Feb 27-Apr 27-Mar 21-May 20-Mar 28-May North-Central Central 25-Feb 21-Apr 3-Mar 28-Apr 25-Feb 27-Apr 17-Mar 19-May 18-Mar 20-May Metro 25-Feb 21-Apr 3-Mar 28-Apr 25-Feb 27-Apr 17-Mar 12-May 18-Mar 13-May South 25-Feb 21-Apr 2-Mar 27-Apr 23-Feb 27-Apr 17-Mar 12-May 15-Mar 20-May Southeast 25-Feb 21-Apr 3-Mar 28-Apr 23-Feb 27-Apr 12-Mar 16-May 15-Mar 20-May 1995 1994 1993 1992 1991 ON OFF ON OFF ON -
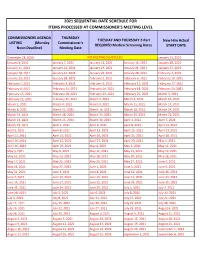
2021 Sequential Date List
2021 SEQUENTIAL DATE SCHEDULE FOR ITEMS PROCESSED AT COMMISSIONER'S MEETING LEVEL COMMISSIONERS AGENDA THURSDAY TUESDAY AND THURSDAY 2-Part New Hire Actual LISTING (Monday Commissioner's REQUIRED Medical Screening Dates START DATE Noon Deadline) Meeting Date December 28, 2020 NO MEETING SCHEDULED January 13, 2021 January 4, 2021 January 7, 2021 January 12, 2021 January 14, 2021 January 20, 2021 January 11, 2021 January 14, 2021 January 19, 2021 January 21, 2021 January 27, 2021 January 18, 2021 January 21, 2021 January 26, 2021 January 28, 2021 February 3, 2021 January 25, 2021 January 28, 2021 February 2, 2021 February 4, 2021 February 10, 2021 February 1, 2021 February 4, 2021 February 9, 2021 February 11, 2021 February 17, 2021 February 8, 2021 February 11, 2021 February 16, 2021 February 18, 2021 February 24, 2021 February 15, 2021 February 18, 2021 February 23, 2021 February 25, 2021 March 3, 2021 February 22, 2021 February 25, 2021 March 2, 2021 March 4, 2021 March 10, 2021 March 1, 2021 March 4, 2021 March 9, 2021 March 11, 2021 March 17, 2021 March 8, 2021 March 11, 2021 March 16, 2021 March 18, 2021 March 24, 2021 March 15, 2021 March 18, 2021 March 23, 2021 March 25, 2021 March 31, 2021 March 22, 2021 March 25, 2021 March 30, 2021 April 1, 2021 April 7, 2021 March 29, 2021 April 1, 2021 April 6, 2021 April 8, 2021 April 14, 2021 April 5, 2021 April 8, 2021 April 13, 2021 April 15, 2021 April 21, 2021 April 12, 2021 April 15, 2021 April 20, 2021 April 22, 2021 April 28, 2021 April 19, 2021 April 22, 2021 April 27, 2021 April -

Pay Date Calendar
Pay Date Information Select the pay period start date that coincides with your first day of employment. Pay Period Pay Period Begins (Sunday) Pay Period Ends (Saturday) Official Pay Date (Thursday)* 1 January 10, 2016 January 23, 2016 February 4, 2016 2 January 24, 2016 February 6, 2016 February 18, 2016 3 February 7, 2016 February 20, 2016 March 3, 2016 4 February 21, 2016 March 5, 2016 March 17, 2016 5 March 6, 2016 March 19, 2016 March 31, 2016 6 March 20, 2016 April 2, 2016 April 14, 2016 7 April 3, 2016 April 16, 2016 April 28, 2016 8 April 17, 2016 April 30, 2016 May 12, 2016 9 May 1, 2016 May 14, 2016 May 26, 2016 10 May 15, 2016 May 28, 2016 June 9, 2016 11 May 29, 2016 June 11, 2016 June 23, 2016 12 June 12, 2016 June 25, 2016 July 7, 2016 13 June 26, 2016 July 9, 2016 July 21, 2016 14 July 10, 2016 July 23, 2016 August 4, 2016 15 July 24, 2016 August 6, 2016 August 18, 2016 16 August 7, 2016 August 20, 2016 September 1, 2016 17 August 21, 2016 September 3, 2016 September 15, 2016 18 September 4, 2016 September 17, 2016 September 29, 2016 19 September 18, 2016 October 1, 2016 October 13, 2016 20 October 2, 2016 October 15, 2016 October 27, 2016 21 October 16, 2016 October 29, 2016 November 10, 2016 22 October 30, 2016 November 12, 2016 November 24, 2016 23 November 13, 2016 November 26, 2016 December 8, 2016 24 November 27, 2016 December 10, 2016 December 22, 2016 25 December 11, 2016 December 24, 2016 January 5, 2017 26 December 25, 2016 January 7, 2017 January 19, 2017 1 January 8, 2017 January 21, 2017 February 2, 2017 2 January -

Due Date Chart 201803281304173331.Xlsx
Special Event Permit Application Due Date Chart for Events from January 1, 2019 - June 30, 2020 If due date lands on a Saturday or Sunday, the due date is moved to the next business day Event Date 30 Calendar days 90 Calendar Days Tuesday, January 01, 2019 Sunday, December 02, 2018 Wednesday, October 03, 2018 Wednesday, January 02, 2019 Monday, December 03, 2018 Thursday, October 04, 2018 Thursday, January 03, 2019 Tuesday, December 04, 2018 Friday, October 05, 2018 Friday, January 04, 2019 Wednesday, December 05, 2018 Saturday, October 06, 2018 Saturday, January 05, 2019 Thursday, December 06, 2018 Sunday, October 07, 2018 Sunday, January 06, 2019 Friday, December 07, 2018 Monday, October 08, 2018 Monday, January 07, 2019 Saturday, December 08, 2018 Tuesday, October 09, 2018 Tuesday, January 08, 2019 Sunday, December 09, 2018 Wednesday, October 10, 2018 Wednesday, January 09, 2019 Monday, December 10, 2018 Thursday, October 11, 2018 Thursday, January 10, 2019 Tuesday, December 11, 2018 Friday, October 12, 2018 Friday, January 11, 2019 Wednesday, December 12, 2018 Saturday, October 13, 2018 Saturday, January 12, 2019 Thursday, December 13, 2018 Sunday, October 14, 2018 Sunday, January 13, 2019 Friday, December 14, 2018 Monday, October 15, 2018 Monday, January 14, 2019 Saturday, December 15, 2018 Tuesday, October 16, 2018 2019 Tuesday, January 15, 2019 Sunday, December 16, 2018 Wednesday, October 17, 2018 Wednesday, January 16, 2019 Monday, December 17, 2018 Thursday, October 18, 2018 Thursday, January 17, 2019 Tuesday, December 18, 2018 -

114-Day Swine Gestation Table
114-DAY SWINE GESTATION TABLE BREEDING DUE BREEDING DUE BREEDING DUE BREEDING DUE BREEDING DUE BREEDING DUE DATE DATE DATE DATE DATE DATE DATE DATE DATE DATE DATE DATE Jan 1 Apr 25 Mar 1 Jun 23 May 1 Aug 23 Jul 1 Oct 23 Sep 1 Dec 24 Nov 1 Feb 23 Jan 2 Apr 26 Mar 2 Jun 24 May 2 Aug 24 Jul 2 Oct 24 Sep 2 Dec 25 Nov 2 Feb 24 Jan 3 Apr 27 Mar 3 Jun 25 May 3 Aug 25 Jul 3 Oct 25 Sep 3 Dec 26 Nov 3 Feb 25 Jan 4 Apr 28 Mar 4 Jun 26 May 4 Aug 26 Jul 4 Oct 26 Sep 4 Dec 27 Nov 4 Feb 26 Jan 5 Apr 29 Mar 5 Jun 27 May 5 Aug 27 Jul 5 Oct 27 Sep 5 Dec 28 Nov 5 Feb 27 Jan 6 Apr 30 Mar 6 Jun 28 May 6 Aug 28 Jul 6 Oct 28 Sep 6 Dec 29 Nov 6 Feb 28 Jan 7 May 1 Mar 7 Jun 29 May 7 Aug 29 Jul 7 Oct 29 Sep 7 Dec 30 Nov 7 Mar 1 Jan 8 May 2 Mar 8 Jun 30 May 8 Aug 30 Jul 8 Oct 30 Sep 8 Dec 31 Nov 8 Mar 2 Jan 9 May 3 Mar 9 Jul 1 May 9 Aug 31 Jul 9 Oct 31 Sep 9 Jan 1 Nov 9 Mar 3 Jan 10 May 4 Mar 10 Jul 2 May 10 Sep 1 Jul 10 Nov 1 Sep 10 Jan 2 Nov 10 Mar 4 Jan 11 May 5 Mar 11 Jul 3 May 11 Sep 2 Jul 11 Nov 2 Sep 11 Jan 3 Nov 11 Mar 5 Jan 12 May 6 Mar 12 Jul 4 May 12 Sep 3 Jul 12 Nov 3 Sep 12 Jan 4 Nov 12 Mar 6 Jan 13 May 7 Mar 13 Jul 5 May 13 Sep 4 Jul 13 Nov 4 Sep 13 Jan 5 Nov 13 Mar 7 Jan 14 May 8 Mar 14 Jul 6 May 14 Sep 5 Jul 14 Nov 5 Sep 14 Jan 6 Nov 14 Mar 8 Jan 15 May 9 Mar 15 Jul 7 May 15 Sep 6 Jul 15 Nov 6 Sep 15 Jan 7 Nov 15 Mar 9 Jan 16 May 10 Mar 16 Jul 8 May 16 Sep 7 Jul 16 Nov 7 Sep 16 Jan 8 Nov 16 Mar 10 Jan 17 May 11 Mar 17 Jul 9 May 17 Sep 8 Jul 17 Nov 8 Sep 17 Jan 9 Nov 17 Mar 11 Jan 18 May 12 Mar 18 Jul 10 May 18 Sep 9 Jul 18 Nov 9 Sep 18 Jan 10 Nov -

Date of Close Contact Exposure
Date of Close Contact Exposure 7 days 10 days 14 days Monday, November 16, 2020 Tuesday, November 24, 2020 Friday, November 27, 2020 Tuesday, December 1, 2020 Tuesday, November 17, 2020 Wednesday, November 25, 2020 Saturday, November 28, 2020 Wednesday, December 2, 2020 Wednesday, November 18, 2020 Thursday, November 26, 2020 Sunday, November 29, 2020 Thursday, December 3, 2020 Thursday, November 19, 2020 Friday, November 27, 2020 Monday, November 30, 2020 Friday, December 4, 2020 Friday, November 20, 2020 Saturday, November 28, 2020 Tuesday, December 1, 2020 Saturday, December 5, 2020 Saturday, November 21, 2020 Sunday, November 29, 2020 Wednesday, December 2, 2020 Sunday, December 6, 2020 Sunday, November 22, 2020 Monday, November 30, 2020 Thursday, December 3, 2020 Monday, December 7, 2020 Monday, November 23, 2020 Tuesday, December 1, 2020 Friday, December 4, 2020 Tuesday, December 8, 2020 Tuesday, November 24, 2020 Wednesday, December 2, 2020 Saturday, December 5, 2020 Wednesday, December 9, 2020 Wednesday, November 25, 2020 Thursday, December 3, 2020 Sunday, December 6, 2020 Thursday, December 10, 2020 Thursday, November 26, 2020 Friday, December 4, 2020 Monday, December 7, 2020 Friday, December 11, 2020 Friday, November 27, 2020 Saturday, December 5, 2020 Tuesday, December 8, 2020 Saturday, December 12, 2020 Saturday, November 28, 2020 Sunday, December 6, 2020 Wednesday, December 9, 2020 Sunday, December 13, 2020 Sunday, November 29, 2020 Monday, December 7, 2020 Thursday, December 10, 2020 Monday, December 14, 2020 Monday, November