Mining for Oxysterols in Cyp7b1-/- Mouse Brain and Plasma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Identification and Developmental Expression of the Full Complement Of
Goldstone et al. BMC Genomics 2010, 11:643 http://www.biomedcentral.com/1471-2164/11/643 RESEARCH ARTICLE Open Access Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish Jared V Goldstone1, Andrew G McArthur2, Akira Kubota1, Juliano Zanette1,3, Thiago Parente1,4, Maria E Jönsson1,5, David R Nelson6, John J Stegeman1* Abstract Background: Increasing use of zebrafish in drug discovery and mechanistic toxicology demands knowledge of cytochrome P450 (CYP) gene regulation and function. CYP enzymes catalyze oxidative transformation leading to activation or inactivation of many endogenous and exogenous chemicals, with consequences for normal physiology and disease processes. Many CYPs potentially have roles in developmental specification, and many chemicals that cause developmental abnormalities are substrates for CYPs. Here we identify and annotate the full suite of CYP genes in zebrafish, compare these to the human CYP gene complement, and determine the expression of CYP genes during normal development. Results: Zebrafish have a total of 94 CYP genes, distributed among 18 gene families found also in mammals. There are 32 genes in CYP families 5 to 51, most of which are direct orthologs of human CYPs that are involved in endogenous functions including synthesis or inactivation of regulatory molecules. The high degree of sequence similarity suggests conservation of enzyme activities for these CYPs, confirmed in reports for some steroidogenic enzymes (e.g. CYP19, aromatase; CYP11A, P450scc; CYP17, steroid 17a-hydroxylase), and the CYP26 retinoic acid hydroxylases. Complexity is much greater in gene families 1, 2, and 3, which include CYPs prominent in metabolism of drugs and pollutants, as well as of endogenous substrates. -

Transcriptomic Characterization of Fibrolamellar Hepatocellular
Transcriptomic characterization of fibrolamellar PNAS PLUS hepatocellular carcinoma Elana P. Simona, Catherine A. Freijeb, Benjamin A. Farbera,c, Gadi Lalazara, David G. Darcya,c, Joshua N. Honeymana,c, Rachel Chiaroni-Clarkea, Brian D. Dilld, Henrik Molinad, Umesh K. Bhanote, Michael P. La Quagliac, Brad R. Rosenbergb,f, and Sanford M. Simona,1 aLaboratory of Cellular Biophysics, The Rockefeller University, New York, NY 10065; bPresidential Fellows Laboratory, The Rockefeller University, New York, NY 10065; cDivision of Pediatric Surgery, Department of Surgery, Memorial Sloan-Kettering Cancer Center, New York, NY 10065; dProteomics Resource Center, The Rockefeller University, New York, NY 10065; ePathology Core Facility, Memorial Sloan-Kettering Cancer Center, New York, NY 10065; and fJohn C. Whitehead Presidential Fellows Program, The Rockefeller University, New York, NY 10065 Edited by Susan S. Taylor, University of California, San Diego, La Jolla, CA, and approved September 22, 2015 (received for review December 29, 2014) Fibrolamellar hepatocellular carcinoma (FLHCC) tumors all carry a exon of DNAJB1 and all but the first exon of PRKACA. This deletion of ∼400 kb in chromosome 19, resulting in a fusion of the produced a chimeric RNA transcript and a translated chimeric genes for the heat shock protein, DNAJ (Hsp40) homolog, subfam- protein that retains the full catalytic activity of wild-type PKA. ily B, member 1, DNAJB1, and the catalytic subunit of protein ki- This chimeric protein was found in 15 of 15 FLHCC patients nase A, PRKACA. The resulting chimeric transcript produces a (21) in the absence of any other recurrent mutations in the DNA fusion protein that retains kinase activity. -

Synonymous Single Nucleotide Polymorphisms in Human Cytochrome
DMD Fast Forward. Published on February 9, 2009 as doi:10.1124/dmd.108.026047 DMD #26047 TITLE PAGE: A BIOINFORMATICS APPROACH FOR THE PHENOTYPE PREDICTION OF NON- SYNONYMOUS SINGLE NUCLEOTIDE POLYMORPHISMS IN HUMAN CYTOCHROME P450S LIN-LIN WANG, YONG LI, SHU-FENG ZHOU Department of Nutrition and Food Hygiene, School of Public Health, Peking University, Beijing 100191, P. R. China (LL Wang & Y Li) Discipline of Chinese Medicine, School of Health Sciences, RMIT University, Bundoora, Victoria 3083, Australia (LL Wang & SF Zhou). 1 Copyright 2009 by the American Society for Pharmacology and Experimental Therapeutics. DMD #26047 RUNNING TITLE PAGE: a) Running title: Prediction of phenotype of human CYPs. b) Author for correspondence: A/Prof. Shu-Feng Zhou, MD, PhD Discipline of Chinese Medicine, School of Health Sciences, RMIT University, WHO Collaborating Center for Traditional Medicine, Bundoora, Victoria 3083, Australia. Tel: + 61 3 9925 7794; fax: +61 3 9925 7178. Email: [email protected] c) Number of text pages: 21 Number of tables: 10 Number of figures: 2 Number of references: 40 Number of words in Abstract: 249 Number of words in Introduction: 749 Number of words in Discussion: 1459 d) Non-standard abbreviations: CYP, cytochrome P450; nsSNP, non-synonymous single nucleotide polymorphism. 2 DMD #26047 ABSTRACT Non-synonymous single nucleotide polymorphisms (nsSNPs) in coding regions that can lead to amino acid changes may cause alteration of protein function and account for susceptivity to disease. Identification of deleterious nsSNPs from tolerant nsSNPs is important for characterizing the genetic basis of human disease, assessing individual susceptibility to disease, understanding the pathogenesis of disease, identifying molecular targets for drug treatment and conducting individualized pharmacotherapy. -

Hepatic Gene Expression of Bile Acid Synthesis Genes from Wild-Type and Fxr−/− Mice
A 2.0 Acox2 B 2.0 Akr1c14 C 2.0 Akr1d1 D 2.0 Amacr ** 1.5 1.5 1.5 1.5 1.0 1.0 1.0 1.0 0.5 0.5 0.5 0.5 mRNA (Fold Change) mRNA mRNA (Fold Change) mRNA mRNA (Fold Change) mRNA mRNA (Fold Change) mRNA 0.0 0.0 0.0 0.0 GSK − 30’ 1h 2h − 30’ 1h 2h GSK − 30’ 1h 2h − 30’ 1h 2h GSK − 30’ 1h 2h − 30’ 1h 2h GSK − 30’ 1h 2h − 30’ 1h 2h FXR WT Fxr−/− FXR WT Fxr−/− FXR WT Fxr−/− FXR WT Fxr−/− E F 2.0 Cyp7b1 2.0 Cyp27a1 G 2.0 Cyp39a1 H 2.0 Hsd3b7 1.5 1.5 1.5 1.5 * 1.0 1.0 1.0 1.0 0.5 0.5 0.5 0.5 mRNA (Fold Change) mRNA mRNA (Fold Change) mRNA mRNA (Fold Change) mRNA mRNA (Fold Change) mRNA 0.0 0.0 0.0 0.0 GSK − 30’ 1h 2h − 30’ 1h 2h GSK − 30’ 1h 2h − 30’ 1h 2h GSK − 30’ 1h 2h − 30’ 1h 2h GSK − 30’ 1h 2h − 30’ 1h 2h FXR WT Fxr−/− FXR WT Fxr−/− FXR WT Fxr−/− FXR WT Fxr−/− I J K 2.0 Hsd17b4 2.0 Scp2 2.0 Slc27a5 L Fxr Cyp7a1 Cyp8b1 1.0 1.5 1.5 1.5 * 1.0 1.0 1.0 0.5 *** *** 0.5 0.5 0.5 mRNA (Fold Change) mRNA mRNA (Fold Change) mRNA mRNA (Fold Change) mRNA mRNA (Fold Change) mRNA *** *** *** 0.0 0.0 0.0 0.0 *** GSK − 30’ 1h 2h − 30’ 1h 2h GSK − 30’ 1h 2h − 30’ 1h 2h GSK − 30’ 1h 2h − 30’ 1h 2h Time (h) 0 4 16 0 4 16 0 4 16 FXR WT Fxr−/− FXR WT Fxr−/− FXR WT Fxr−/− post plating M N CYP7A1 CYP8B1 Supplementary Figure 1 – FXR activation leads to rapid changes in gene expression 1.0 1.0 (A-K) Hepatic gene expression of bile acid synthesis genes from wild-type and Fxr−/− mice. -

Polyclonal Antibody to CYP7B1 (C-Term) - Aff - Purified
OriGene Technologies, Inc. OriGene Technologies GmbH 9620 Medical Center Drive, Ste 200 Schillerstr. 5 Rockville, MD 20850 32052 Herford UNITED STATES GERMANY Phone: +1-888-267-4436 Phone: +49-5221-34606-0 Fax: +1-301-340-8606 Fax: +49-5221-34606-11 [email protected] [email protected] AP20144PU-N Polyclonal Antibody to CYP7B1 (C-term) - Aff - Purified Alternate names: 25-hydroxycholesterol 7-alpha-hydroxylase, Cytochrome P450 7B1, Oxysterol 7-alpha- hydroxylase Quantity: 0.1 mg Concentration: 0.5 mg/ml Background: P450 enzymes constitute a family of monooxygenase enzymes that are involved in the metabolism of a wide array of endogenous and xenobiotic compounds including cholesterol. CYP8B1 moderates the ratio of cholic acid over chenodeoxycholic acid to control the solubility of cholesterol. P450 cholesterol 7-hydroxylase, CYP7A1, is the rate limiting enzyme of bile acid synthesis in the liver, and its expression is mediated by the bile acid receptor FXR. CYP27A1 catalyzes vitamin D3 25-hydroxylation and is localized to the mitochondria in kidney and liver. CYP7B1 (oxysterol 7-α-hydroxylase) functions as an enzyme in the alternate bile acid synthesis pathway. Specifically, CYP7B1 metabolizes 25-and 27-hydroxycholesterol. The gene encoding human CYP7B1 maps to chromosome 8q21.3. Mutations in the CYP7B1 gene may cause a metabolic defect in bile acid synthesis characterized by elevated urinary bile acid excretion, severe cholestasis, cirrhosis and liver synthetic failure. Uniprot ID: O75881 NCBI: NP_004811 GeneID: 9420 Host: Goat Immunogen: Peptide with sequence C-YPDSDVLFRYKVKS, from the C Terminus of the protein sequence according to NP_004811.1. Format: State: Liquid purified IgG fraction. -

Metabolic Activation and Toxicological Evaluation of Polychlorinated Biphenyls in Drosophila Melanogaster T
www.nature.com/scientificreports OPEN Metabolic activation and toxicological evaluation of polychlorinated biphenyls in Drosophila melanogaster T. Idda1,7, C. Bonas1,7, J. Hofmann1, J. Bertram1, N. Quinete1,2, T. Schettgen1, K. Fietkau3, A. Esser1, M. B. Stope4, M. M. Leijs3, J. M. Baron3, T. Kraus1, A. Voigt5,6 & P. Ziegler1* Degradation of polychlorinated biphenyls (PCBs) is initiated by cytochrome P450 (CYP) enzymes and includes PCB oxidation to OH-metabolites, which often display a higher toxicity than their parental compounds. In search of an animal model refecting PCB metabolism and toxicity, we tested Drosophila melanogaster, a well-known model system for genetics and human disease. Feeding Drosophila with lower chlorinated (LC) PCB congeners 28, 52 or 101 resulted in the detection of a human-like pattern of respective OH-metabolites in fy lysates. Feeding fies high PCB 28 concentrations caused lethality. Thus we silenced selected CYPs via RNA interference and analyzed the efect on PCB 28-derived metabolite formation by assaying 3-OH-2′,4,4′-trichlorobiphenyl (3-OHCB 28) and 3′-OH-4′,4,6′-trichlorobiphenyl (3′-OHCB 28) in fy lysates. We identifed several drosophila CYPs (dCYPs) whose knockdown reduced PCB 28-derived OH-metabolites and suppressed PCB 28 induced lethality including dCYP1A2. Following in vitro analysis using a liver-like CYP-cocktail, containing human orthologues of dCYP1A2, we confrm human CYP1A2 as a PCB 28 metabolizing enzyme. PCB 28-induced mortality in fies was accompanied by locomotor impairment, a common phenotype of neurodegenerative disorders. Along this line, we show PCB 28-initiated caspase activation in diferentiated fy neurons. -

Alteration of the Steroidogenesis in Boys with Autism Spectrum Disorders
Janšáková et al. Translational Psychiatry (2020) 10:340 https://doi.org/10.1038/s41398-020-01017-8 Translational Psychiatry ARTICLE Open Access Alteration of the steroidogenesis in boys with autism spectrum disorders Katarína Janšáková 1, Martin Hill 2,DianaČelárová1,HanaCelušáková1,GabrielaRepiská1,MarieBičíková2, Ludmila Máčová2 and Daniela Ostatníková1 Abstract The etiology of autism spectrum disorders (ASD) remains unknown, but associations between prenatal hormonal changes and ASD risk were found. The consequences of these changes on the steroidogenesis during a postnatal development are not yet well known. The aim of this study was to analyze the steroid metabolic pathway in prepubertal ASD and neurotypical boys. Plasma samples were collected from 62 prepubertal ASD boys and 24 age and sex-matched controls (CTRL). Eighty-two biomarkers of steroidogenesis were detected using gas-chromatography tandem-mass spectrometry. We observed changes across the whole alternative backdoor pathway of androgens synthesis toward lower level in ASD group. Our data indicate suppressed production of pregnenolone sulfate at augmented activities of CYP17A1 and SULT2A1 and reduced HSD3B2 activity in ASD group which is partly consistent with the results reported in older children, in whom the adrenal zona reticularis significantly influences the steroid levels. Furthermore, we detected the suppressed activity of CYP7B1 enzyme readily metabolizing the precursors of sex hormones on one hand but increased anti-glucocorticoid effect of 7α-hydroxy-DHEA via competition with cortisone for HSD11B1 on the other. The multivariate model found significant correlations between behavioral indices and circulating steroids. From dependent variables, the best correlation was found for the social interaction (28.5%). Observed changes give a space for their utilization as biomarkers while reveal the etiopathogenesis of ASD. -

Novel Copy-Number Variations in Pharmacogenes Contribute to Interindividual Differences in Drug Pharmacokinetics
ORIGINAL RESEARCH ARTICLE © American College of Medical Genetics and Genomics Novel copy-number variations in pharmacogenes contribute to interindividual differences in drug pharmacokinetics María Santos, MSc1, Mikko Niemi, PhD2, Masahiro Hiratsuka, PhD3, Masaki Kumondai, BSc3, Magnus Ingelman-Sundberg, PhD4, Volker M. Lauschke, PhD4 and Cristina Rodríguez-Antona, PhD1,5 Purpose: Variability in pharmacokinetics and drug response is of the genes studied. We experimentally confirmed novel deletions shaped by single-nucleotide variants (SNVs) as well as copy- in CYP2C19, CYP4F2, and SLCO1B3 by Sanger sequencing and number variants (CNVs) in genes with importance for drug validated their allelic frequencies in selected populations. absorption, distribution, metabolism, and excretion (ADME). Conclusion: CNVs are an additional source of pharmacogenetic While SNVs have been extensively studied, a systematic assessment variability with important implications for drug response and of the CNV landscape in ADME genes is lacking. personalized therapy. This, together with the important contribu- Methods: We integrated data from 2,504 whole genomes from the tion of rare alleles to the variability of pharmacogenes, emphasizes 1000 Genomes Project and 59,898 exomes from the Exome the necessity of comprehensive next-generation sequencing–based Aggregation Consortium to identify CNVs in 208 relevant genotype identification for an accurate prediction of the genetic pharmacogenes. variability of drug pharmacokinetics. Results: We describe novel exonic deletions -

Widespread Signals of Convergent Adaptation to High Altitude in Asia and America
bioRxiv preprint doi: https://doi.org/10.1101/002816; this version posted September 26, 2014. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Widespread signals of convergent adaptation to high altitude in Asia and America Matthieu Foll 1,2,3,*, Oscar E. Gaggiotti 4,5, Josephine T. Daub 1,2, Alexandra Vatsiou 5 and Laurent Excoffier 1,2 1 CMPG, Institute oF Ecology and Evolution, University oF Berne, Berne, 3012, Switzerland 2 Swiss Institute oF BioinFormatics, Lausanne, 1015, Switzerland 3 Present address: School oF LiFe Sciences, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, 1015, Switzerland 4 School oF Biology, Scottish Oceans Institute, University oF St Andrews, St Andrews, FiFe, KY16 8LB, UK 5 Laboratoire d'Ecologie Alpine (LECA), UMR 5553 CNRS-Université de Grenoble, Grenoble, France * Corresponding author: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/002816; this version posted September 26, 2014. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Abstract Living at high-altitude is one oF the most diFFicult challenges that humans had to cope with during their evolution. Whereas several genomic studies have revealed some oF the genetic bases oF adaptations in Tibetan, Andean and Ethiopian populations, relatively little evidence oF convergent evolution to altitude in diFFerent continents has accumulated. -

Expression of CYP2S1 in Human Hepatic Stellate Cells
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector FEBS Letters 581 (2007) 781–786 Expression of CYP2S1 in human hepatic stellate cells Carylyn J. Mareka, Steven J. Tuckera, Matthew Korutha, Karen Wallacea,b, Matthew C. Wrighta,b,* a School of Medical Sciences, Institute of Medical Science, University of Aberdeen, Aberdeen, UK b Liver Faculty Research Group, School of Clinical and Laboratory Sciences, University of Newcastle, Newcastle, UK Received 22 November 2006; revised 16 January 2007; accepted 23 January 2007 Available online 2 February 2007 Edited by Laszlo Nagy the expression and accumulation of scarring extracellular Abstract Activated stellate cells are myofibroblast-like cells associated with the generation of fibrotic scaring in chronically fibrotic matrix protein [2]. It is currently thought an inhibition damaged liver. Gene chip analysis was performed on cultured fi- of fibrosis in liver diseases may be an effective approach to brotic stellate cells. Of the 51 human CYP genes known, 13 treating patients for which the cause is refractive to current CYP and 5 CYP reduction-related genes were detected with 4 treatments (e.g. in approx. 30% of hepatitis C infected CYPs (CYP1A1, CYP2E1, CY2S1 and CYP4F3) consistently patients) [2,3]. At present, there is no approved treatment for present in stellate cells isolated from three individuals. Quantita- fibrosis. tive RT-PCR indicated that CYP2S1 was a major expressed Inadvertent toxicity of drugs is often associated with a ‘‘met- CYP mRNA transcript. The presence of a CYP2A-related pro- abolic activation’’ by CYPs [1]. -

Glyphosate's Suppression of Cytochrome P450 Enzymes
Entropy 2013, 15, 1416-1463; doi:10.3390/e15041416 OPEN ACCESS entropy ISSN 1099-4300 www.mdpi.com/journal/entropy Review Glyphosate’s Suppression of Cytochrome P450 Enzymes and Amino Acid Biosynthesis by the Gut Microbiome: Pathways to Modern Diseases Anthony Samsel 1 and Stephanie Seneff 2,* 1 Independent Scientist and Consultant, Deerfield, NH 03037, USA; E-Mail: [email protected] 2 Computer Science and Artificial Intelligence Laboratory, MIT, Cambridge, MA 02139, USA * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-617-253-0451; Fax: +1-617-258-8642. Received: 15 January 2013; in revised form: 10 April 2013 / Accepted: 10 April 2013 / Published: 18 April 2013 Abstract: Glyphosate, the active ingredient in Roundup®, is the most popular herbicide used worldwide. The industry asserts it is minimally toxic to humans, but here we argue otherwise. Residues are found in the main foods of the Western diet, comprised primarily of sugar, corn, soy and wheat. Glyphosate's inhibition of cytochrome P450 (CYP) enzymes is an overlooked component of its toxicity to mammals. CYP enzymes play crucial roles in biology, one of which is to detoxify xenobiotics. Thus, glyphosate enhances the damaging effects of other food borne chemical residues and environmental toxins. Negative impact on the body is insidious and manifests slowly over time as inflammation damages cellular systems throughout the body. Here, we show how interference with CYP enzymes acts synergistically with disruption of the biosynthesis of aromatic amino acids by gut bacteria, as well as impairment in serum sulfate transport. Consequences are most of the diseases and conditions associated with a Western diet, which include gastrointestinal disorders, obesity, diabetes, heart disease, depression, autism, infertility, cancer and Alzheimer’s disease. -
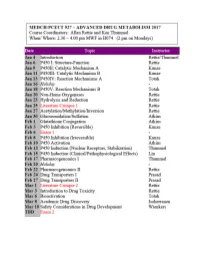
CYTOCHROME P450: Structure-Function
MEDCH 527 AER Jan. 4-6, 2017 CYTOCHROME P450: Structure-Function 1. General P450 Characteristics anD Taxonomy 2. Human P450s – Substrate anD Inhibitor Selectivities 3. Structure-Function Aspects of LiganD BinDing, P450 ReDuction anD Oxygen Activation References P450 Homepage -http:// http://drnelson.uthsc.edu/CytochromeP450.html Nelson DR et al., Pharmacogenetics. 1996 6(1):1-42: Nelson DR et al., Pharmacogenetics. 2004 14(1):1-18. Testa, B. The Biochemistry of Drug Metabolism: A 6 Part Series in Chem. BioDivers. (2006-2008). Sligar, SG. Glimpsing the critical intermediate in cytochrome P450 oxidations. Science. 2010 Nov 12;330(6006):924-5. Johnson EF, et al. Correlating structure and function of drug metabolizing enzymes:Progress and ongoing challenges. Drug Metab. Dispos. 42:9-22 (2014). Zientek MA and Youdim K, Reaction Phenotyping:Advances in experimental strategies used to characterize the contribution of drug metabolizing enzymes. Drug Metab. Dispos. 43:163-181 (2015). Almazroo et al., Drug Metabolism in the Liver. Clin. Liver Dis. 21:1-20 (2017). Absorption, Distribution, Metabolism and Excretion (ADME) of Orally Administered Drugs To reach their sites of action in the body, orally administered drugs must be absorbed from the small intestine, survive first pass metabolism - typically in the liver - before eventually being excreted, usually as drug metabolites in the bile and kidney. Therefore, clinically useful drugs must be able to cross an array of cell membranes, which are composed of a lipid bilayer. Drugs must exhibit an adequate degree of lipophilicity (logP of ~2-4) in order to able to dissolve into this lipoidal environment. Many drug metabolism processes render lipophilic drugs more water-soluble so as to facilitate excretion via the kidneys and bile.