An Ecological Study of Hunting Creek
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table of Contents
Photo by King Montgomery. by Photo Table of Contents 7 Foreword 8 Fly Fishing Virginia 11 Flies to Use in Virginia 17 Top Virginia Fly Fishing Waters 19 Accotink Creek 21 Back Creek 25 Big Wilson Creek 29 Briery Creek Lake 33 Chesapeake Bay Islands Beasley. Beau by Photo 37 Conway River 41 Dragon Run 43 Gwynn Island 47 Holmes Run Beasley. Beau by Photo 51 Holston River, South Fork 53 Jackson River, Lower Section 59 Jackson River, Upper Section 63 James River, Lower Section 67 James River, Upper Section 71 Lake Brittle 75 Lynnhaven River, Bay, & Inlet 79 Maury River 4 Photo by Eric Evans. Eric by Photo 83 Mossy Creek 87 New River, Lower Section 91 New River, Upper Section 95 North Creek 97 Passage Creek 99 Piankatank River 101 Rapidan River, Lower Section Beasley. Beau by Photo 105 Rapidan River, Upper Section 109 Rappahannock River, Lower Section 113 Rappahannock River, Upper Section 117 Rivanna River 121 Rose River 125 Rudee Inlet 129 Shenandoah River, North Fork Photo by Beau Beasley. Beau by Photo 133 Shenandoah River, South Fork 137 South River 141 St. Mary’s River 145 Whitetop Laurel Creek Chris Newsome. by Photo 148 Private Waters 151 Resources 155 Conservation 156 Other No Nonsense Guides 158 Fly Fishing Knots 5 Arlington 81 66 Interstate South U.S. Highway River 95 State Highway 81 Other Roadway 64 64 Richmond Virginia Boat Launch 64 460 Fish Hatchery Roanoke Hampton 81 95 To Campground 77 58 Hermitage 254 To Grottoes To 340 Staunton ay rkw n Pa ema Hop 254 ver Ri 250 h ut So 340 340 1 Waynesboro To 2 Staunton 2 3 664 64 624 To Charlottesville 250 er iv R h ut 64 So 624 To 1 Constitution Park–Home of Charlottesville Virginia Fly Fishing Festival 2 Good Wading 3 Low water dam South River 136 South River outh River is one of the most underrated fisheries in the Old Dominion. -

2E – Four Mile
City of Alexandria, Virginia Geologic Atlas of the City of Alexandria, Virginia and Vicinity – Plate 2E NW E GEOLOGIC CROSS SECTION E’ SE FEET NORTHWEST FEET Claremont FOUR MILE RUN 200 GT-112 200 191 Tcg by Anthony H. Fleming, 2015 173 Kpl Barcroft Kpb Park 150 151 150 Kpcv 89 Kpcc Kpcs 100 100 Qc Lucky BEVERLEY HILLS Reservoir Qs Run Charles Kpcg Qaf SHIRLEY HWY Barrett Woods Qa QUAKER LANE MOUNT IDA J-1 60 School 63 GT-42 55 GT-85 GT-185 Shirlington GT-62 50 Qa 48 50 47 POTOMAC YARDS 50 GT-68 GT-67 Qto Qcf Qt 45 44 Qt Arlandria Qcf 39 38 Qa 39 38 Four Mile Rte 1 GT-4 Qcf 30 31 32 Hume Lynhaven 30 Kpcc 30 28 s Qt 25 Run Park GT-136 Qcf Spring GT-117 OCi Kpcv Qto 20 17 15 F-3 Kpcv 16 af 11 Qto af Qa af Qto Kpcs Kpcc Kpcc Kpch Qs Qt 3 SL 0 Qe SL -3 -7 ? Qal Qto-c Kpcc org -25 95% sand org Qto-c -38 -50 Kpch? -50 Kpcc Kpcc 58% total sand (96/164) -68 OCI Kpcs -100 Ogu -100 35% sand Kpcv? -120 Kpcc -150 -149 -150 OCs Kpcs? -200 -195 -200 RCSZ -250 -250 -300 -300 SEE PLATE 5 FOR EXPLANATION OF MAP UNITS VERTICAL EXAGGERATION 20X 1000 0 1000 2000 3000 4000 5000 6000 7000 8000 9000 10000 FEET EXPLANATION OF CROSS SECTION SYMBOLS: WATER WELL GEOTECHNICAL BORING SITES WATER LEVELS REPORTED IN WELLS OTHER SYMBOLS J-60 WELL ID NUMBER AND SURFACE ELEVATION GT-27 ID NUMBER AND HIGHEST AND GEOTECHNICAL BORINGS 250 222 (SOURCE: J-JOHNSTON; D-DARTON; F-FROELICH) SURFACE ELEVATION 47 SURFACE EXPOSURE. -

Nomination Form
NPS Form 10-900 OMB No. 1024-0018 United States Department of the Interior National Park Service National Register of Historic Places Registration Form This form is for use in nominating or requesting determinations for individual properties and districts. See instructions in National Register Bulletin, How to Complete the National Register of Historic Places Registration Form. If any item does not apply to the property being documented, enter "N/A" for "not applicable." For functions, architectural classification, materials, and areas of significance, enter only categories and subcategories from the instructions. 1. Name of Property Historic name: Woodlawn Cultural Landscape Historic District Other names/site number: DHR File No.: 029-5181 Name of related multiple property listing: N/A (Enter "N/A" if property is not part of a multiple property listing ____________________________________________________________________________ 2. Location Street & number: Bounded by Old Mill Rd, Mt Vernon Memorial Hwy, Fort Belvoir, and Dogue Creek City or town: Alexandria State: VA County: Fairfax Not For Publication: N/A Vicinity: X ____________________________________________________________________________ 3. State/Federal Agency Certification As the designated authority under the National Historic Preservation Act, as amended, I hereby certify that this X nomination ___ request for determination of eligibility meets the documentation standards for registering properties in the National Register of Historic Places and meets the procedural and professional -

Native Vascular Flora of the City of Alexandria, Virginia
Native Vascular Flora City of Alexandria, Virginia Photo by Gary P. Fleming December 2015 Native Vascular Flora of the City of Alexandria, Virginia December 2015 By Roderick H. Simmons City of Alexandria Department of Recreation, Parks, and Cultural Activities, Natural Resources Division 2900-A Business Center Drive Alexandria, Virginia 22314 [email protected] Suggested citation: Simmons, R.H. 2015. Native vascular flora of the City of Alexandria, Virginia. City of Alexandria Department of Recreation, Parks, and Cultural Activities, Alexandria, Virginia. 104 pp. Table of Contents Abstract ............................................................................................................................................ 2 Introduction ...................................................................................................................................... 2 Climate ..................................................................................................................................... 2 Geology and Soils .................................................................................................................... 3 History of Botanical Studies in Alexandria .............................................................................. 5 Methods ............................................................................................................................................ 7 Results and Discussion .................................................................................................................... -

Pohick Creek Watershed Management Plan Are Included in This Section
Watershed Management Area Restoration Strategies 5.0 Watershed Management Area Restoration Strategies The Pohick Creek Watershed is divided into ten smaller watershed management areas (WMAs) based on terrain. Summaries of Pohick Creek’s ten WMAs are listed in the following WMA sections, including field reconnaissance findings, existing and future land use, stream conditions and stormwater infrastructure. For Fairfax County planning and management purposes the WMAs have been further subdivided into smaller subwatersheds. These areas, typically 100 – 300 acres, were used as the basic units for modeling and other evaluations. Each WMA was examined at the subwatershed level in order to capture as much data as possible. The subwatershed conditions were reviewed and problem areas were highlighted. Projects were proposed in problematic subwatersheds. The full Pohick Creek Draft Watershed Workbook, which contains detailed watershed characterizations, can be found in the Technical Appendices. Pohick Creek has four major named tributaries (see Map 3-1.1 in Chapter 3). In the northern portions of the watershed two main tributaries converge into Pohick Creek stream. The Rabbit Branch tributary begins in the highly developed areas of George Mason University and Fairfax City, while Sideburn Branch tributary begins in the highly developed area southwest of George Mason University. The confluence of these two headwater tributaries forms the Pohick Creek main stem. The Middle Run tributary drains Huntsman Lake and moderately-developed residential areas. The South Run tributary drains Burke Lake and Lake Mercer, as well as the low-density southwestern portion of the watershed. The restoration strategies proposed to be implemented within the next ten years (0 – 10-year plan) consist of 90 structural projects. -
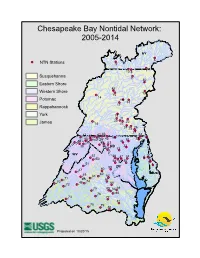
Chesapeake Bay Nontidal Network: 2005-2014
Chesapeake Bay Nontidal Network: 2005-2014 NY 6 NTN Stations 9 7 10 8 Susquehanna 11 82 Eastern Shore 83 Western Shore 12 15 14 Potomac 16 13 17 Rappahannock York 19 21 20 23 James 18 22 24 25 26 27 41 43 84 37 86 5 55 29 85 40 42 45 30 28 36 39 44 53 31 38 46 MD 32 54 33 WV 52 56 87 34 4 3 50 2 58 57 35 51 1 59 DC 47 60 62 DE 49 61 63 71 VA 67 70 48 74 68 72 75 65 64 69 76 66 73 77 81 78 79 80 Prepared on 10/20/15 Chesapeake Bay Nontidal Network: All Stations NTN Stations 91 NY 6 NTN New Stations 9 10 8 7 Susquehanna 11 82 Eastern Shore 83 12 Western Shore 92 15 16 Potomac 14 PA 13 Rappahannock 17 93 19 95 96 York 94 23 20 97 James 18 98 100 21 27 22 26 101 107 24 25 102 108 84 86 42 43 45 55 99 85 30 103 28 5 37 109 57 31 39 40 111 29 90 36 53 38 41 105 32 44 54 104 MD 106 WV 110 52 112 56 33 87 3 50 46 115 89 34 DC 4 51 2 59 58 114 47 60 35 1 DE 49 61 62 63 88 71 74 48 67 68 70 72 117 75 VA 64 69 116 76 65 66 73 77 81 78 79 80 Prepared on 10/20/15 Table 1. -

Authorization to Discharge Under the Virginia Stormwater Management Program and the Virginia Stormwater Management Act
COMMONWEALTHof VIRGINIA DEPARTMENTOFENVIRONMENTAL QUALITY Permit No.: VA0088587 Effective Date: April 1, 2015 Expiration Date: March 31, 2020 AUTHORIZATION TO DISCHARGE UNDER THE VIRGINIA STORMWATER MANAGEMENT PROGRAM AND THE VIRGINIA STORMWATER MANAGEMENT ACT Pursuant to the Clean Water Act as amended and the Virginia Stormwater Management Act and regulations adopted pursuant thereto, the following owner is authorized to discharge in accordance with the effluent limitations, monitoring requirements, and other conditions set forth in this state permit. Permittee: Fairfax County Facility Name: Fairfax County Municipal Separate Storm Sewer System County Location: Fairfax County is 413.15 square miles in area and is bordered by the Potomac River to the East, the city of Alexandria and the county of Arlington to the North, the county of Loudoun to the West, and the county of Prince William to the South. The owner is authorized to discharge from municipal-owned storm sewer outfalls to the surface waters in the following watersheds: Watersheds: Stormwater from Fairfax County discharges into twenty-two 6lh order hydrologic units: Horsepen Run (PL18), Sugarland Run (PL21), Difficult Run (PL22), Potomac River- Nichols Run-Scott Run (PL23), Potomac River-Pimmit Run (PL24), Potomac River- Fourmile Run (PL25), Cameron Run (PL26), Dogue Creek (PL27), Potomac River-Little Hunting Creek (PL28), Pohick Creek (PL29), Accotink Creek (PL30),(Upper Bull Run (PL42), Middle Bull Run (PL44), Cub Run (PL45), Lower Bull Run (PL46), Occoquan River/Occoquan Reservoir (PL47), Occoquan River-Belmont Bay (PL48), Potomac River- Occoquan Bay (PL50) There are 15 major streams: Accotink Creek, Bull Run, Cameron Run (Hunting Creek), Cub Run, Difficult Run, Dogue Creek, Four Mile Run, Horsepen Run, Little Hunting Creek, Little Rocky Run, Occoquan Receiving Streams: River, Pimmit run, Pohick creek, Popes Head Creek, Sugarland Run, and various other minor streams. -

River Watch Spring 2010
The Newsletter of Potomac RiveRkeepeR, Inc. Volume 7, Issue 1, Winter 2010 495 HOT Lanes Construction Polluting In This Issue Accotink Creek Agricultural Pollution in W. Virginia page 2 s snow pummeled northern Virginia, APotomac Riverkeeper took action against a major polluter in Fairfax Stormwater Regulations Stalled County, VA. page 3 As you might know, a portion of the I- 495 High Occupancy Toll (“HOT”) Lanes From the Board construction site is severely damaging page 4 Accotink Creek, the Potomac River, and the Chesapeake Bay. Sediment pollution News in Brief is leaving the site and has entered page 5 Accotink Creek and its tributaries on numerous occasions. Potomac Riverkeeper’s 10th Anniversary Potomac Riverkeeper and two individuals page 6 sought to end this problem by notifying Fluor-Lane LLC, the HOT Lanes developers, of our intent to sue under the Clean Water Upcoming Events Act (CWA) if Fluor-Lane continues to violate page 7 Virginia law and allow the pollution to enter Accotink Creek. Coverage of our Mattawoman WWTP Permit action ran in The Washington Post. page 8 Flour-Lane has not stopped polluting despite numerous complaints from the public and inspections from state Polluted water is leaving the HOT Lanes Get the DIRT Out agencies. If Fluor-Lane does not stop the construction site and entering Accotink Creek. Photo by Kris Unger. As you just read, some developers allow polluted sludge pollution and comply with the law, legal to run into our rivers and streams, leaving taxpayers action may be one of the few remaining the stream. He also made site visits and with a hefty clean up bill. -

A Direct Route to George Washington's Home
Office of Historic Alexandria City of Alexandria, Virginia Out of the Attic A direct route to George Washington’s home Alexandria Times, February 12, 2015 Image: Great Hunting Creek. Photo, Office of Historic Alexandria. his week, we continue our discussion of the man-made changes to Great Hunting Creek T and Cameron Run. With the construction of the Richmond Highway causeway across the creek in 1809 and then the building of a streetcar rail bridge across the wide mouth of the waterway at the turn of the 20th century, impediments to proper drainage were well in place by 1900. But within 30 years, a major new construction project would replace the elevated rail bridge with a permanent land form, further restricting the ebb and flow of natural currents and drainage flow far upstream. At the time the streetcar rail bridge was under construction, an Alexandria business group first proposed a new roadway to link the nation’s capital with the tourist haven, Mount Vernon, 15 miles away. As projected, the new highway would cross the Potomac River near Arlington House, as well as at Great Hunting Creek on suspension bridges high above the waterways, named the “Memorial Bridges” in honor of the Civil War dead and to reflect the reunification of the Union and Confederate states. In the rural wilderness of Fairfax County, south of Alexandria, the highway would include 13 rest areas, each featuring a large pavilion reflecting one of the original colonies, with landscaping, architecture and exhibits appropriate to the earliest states. By the late 1920s, Congress finally authorized construction of the George Washington Memorial Parkway, in honor of the upcoming 200th birthday of the nation’s first president in 1932 as promoted by Alexandria businessmen, albeit without the more lavish plans espoused nearly three decades earlier. -

Belle Haven, Dogue Creek and Four Mile Run
1 Introduction to Watersheds A watershed is an area of land that drains all of its water to a specific lake or river. As rainwater and melting snow run downhill, they carry sediment and other materials into our streams, lakes, wetlands and groundwater. The boundary of a watershed is defined by the watershed divide, which is the ridge of highest elevation surrounding a given stream or network of streams. A drop of rainwater falling outside of this boundary will enter a different watershed and will flow to a different body of water. Figure 1-1: Diagram of a watershed Streams and rivers may flow through many different types of land use in their paths to the ocean. In the above illustration from the U.S. Environmental Protection Agency, water flows from agricultural lands to residential areas to industrial zones as it moves downstream. Each land use presents unique impacts and challenges on water quality. The size of a watershed can be subjective; it depends on the scale that is being considered. The image to the left depicts the extent of the Chesapeake Bay watershed, "the big picture" that is linked to our local concerns. This watershed covers 64,000 square miles and crosses into six states: New York, Pennsylvania, Delaware, West Virginia, Maryland, Virginia and the District of Columbia. One of the watersheds that comprise the Chesapeake Bay watershed is the Potomac River watershed. Fairfax County, as shown on the map, occupies approximately 400 square miles of the Potomac River watershed. This area contains 30 smaller watersheds. Think of watersheds as being "nested" within each successively larger one. -

Brook Trout Outcome Management Strategy
Brook Trout Outcome Management Strategy Introduction Brook Trout symbolize healthy waters because they rely on clean, cold stream habitat and are sensitive to rising stream temperatures, thereby serving as an aquatic version of a “canary in a coal mine”. Brook Trout are also highly prized by recreational anglers and have been designated as the state fish in many eastern states. They are an essential part of the headwater stream ecosystem, an important part of the upper watershed’s natural heritage and a valuable recreational resource. Land trusts in West Virginia, New York and Virginia have found that the possibility of restoring Brook Trout to local streams can act as a motivator for private landowners to take conservation actions, whether it is installing a fence that will exclude livestock from a waterway or putting their land under a conservation easement. The decline of Brook Trout serves as a warning about the health of local waterways and the lands draining to them. More than a century of declining Brook Trout populations has led to lost economic revenue and recreational fishing opportunities in the Bay’s headwaters. Chesapeake Bay Management Strategy: Brook Trout March 16, 2015 - DRAFT I. Goal, Outcome and Baseline This management strategy identifies approaches for achieving the following goal and outcome: Vital Habitats Goal: Restore, enhance and protect a network of land and water habitats to support fish and wildlife, and to afford other public benefits, including water quality, recreational uses and scenic value across the watershed. Brook Trout Outcome: Restore and sustain naturally reproducing Brook Trout populations in Chesapeake Bay headwater streams, with an eight percent increase in occupied habitat by 2025. -

Corridor Analysis for the Potomac Heritage National Scenic Trail in Northern Virginia
Corridor Analysis For The Potomac Heritage National Scenic Trail In Northern Virginia June 2011 Acknowledgements The Northern Virginia Regional Commission (NVRC) wishes to acknowledge the following individuals for their contributions to this report: Don Briggs, Superintendent of the Potomac Heritage National Scenic Trail for the National Park Service; Liz Cronauer, Fairfax County Park Authority; Mike DePue, Prince William Park Authority; Bill Ference, City of Leesburg Park Director; Yon Lambert, City of Alexandria Department of Transportation; Ursula Lemanski, Rivers, Trails and Conservation Assistance Program for the National Park Service; Mark Novak, Loudoun County Park Authority; Patti Pakkala, Prince William County Park Authority; Kate Rudacille, Northern Virginia Regional Park Authority; Jennifer Wampler, Virginia Department of Conservation and Recreation; and Greg Weiler, U.S. Fish and Wildlife Service. The report is an NVRC staff product, supported with funds provided through a cooperative agreement with the National Capital Region National Park Service. Any assessments, conclusions, or recommendations contained in this report represent the results of the NVRC staff’s technical investigation and do not represent policy positions of the Northern Virginia Regional Commission unless so stated in an adopted resolution of said Commission. The views expressed in this document are those of the authors and do not necessarily reflect the views of the jurisdictions, the National Park Service, or any of its sub agencies. Funding for this report was through a cooperative agreement with The National Park Service Report prepared by: Debbie Spiliotopoulos, Senior Environmental Planner Northern Virginia Regional Commission with assistance from Samantha Kinzer, Environmental Planner The Northern Virginia Regional Commission 3060 Williams Drive, Suite 510 Fairfax, VA 22031 703.642.0700 www.novaregion.org Page 2 Northern Virginia Regional Commission As of May 2011 Chairman Hon.