Marine Baitfish Culture
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Xiphopenaeus Kroyeri
unuftp.is Final Project 2018 Sustainable Management of Guyana’s Seabob (Xiphopenaeus kroyeri.) Trawl Fishery Seion Adika Richardson Ministry of Agriculture, Fisheries Department Co-operative Republic of Guyana [email protected] Supervisors: Dr. Pamela J. Woods Dr. Ingibjörg G. Jónsdóttir Marine and Freshwater Research Institute Iceland [email protected] [email protected] ABSTRACT Seabob (Xiphopenaeus kroyeri) is the most exploited shrimp species in Guyana and the largest seafood export. This species is mostly caught by seabob trawlers, sometimes with large quantities of bycatch. The goal of this paper is to promote the long-term sustainability of marine stocks impacted by this fishery, by analysing 1) shrimp stock status, 2) the current state of knowledge regarding bycatch impacts, and 3) spatial fishing patterns of seabob trawlers. To address the first, the paper discusses a stock assessment on Guyana`s seabob stock using the Stochastic Surplus Production Model in Continuous-Time (SPiCT). The model output suggests that the stock is currently in an overfished state, i.e., that the predicted Absolute Stock Biomass (Bt) for 2018 is four times smaller than the Biomass which yields Maximum Sustainable Yield at equilibrium (BMSY) and the current fishing mortality (Ft) is six times above the required to achieve Fishing Mortality which results in Maximum Sustainable Yield at equilibrium (FMSY). These results indicate a more overfished state than was generated by the previous stock assessment which concluded that the stock was fully exploited but not overfished (Medley, 2013).To address the second goal, the study linked catch and effort data with spatial Vessel Monitoring System (VMS) data to analyse the mixture of target and non-target species within the seabob fishery. -

Year 2 Data Summary Report: Nekton of Sarasota Bay and a Comparison of Nekton Community Structure in Adjacent Southwest Florida Estuaries
Year 2 Data Summary Report: Nekton of Sarasota Bay and a Comparison of Nekton Community Structure in Adjacent Southwest Florida Estuaries T.C. MacDonald; E. Weather; R.F. Jones; R.H. McMichael, Jr. Florida Fish and Wildlife Conservation Commission Fish and Wildlife Research Institute 100 Eighth Avenue Southeast St. Petersburg, Florida 33701-5095 Prepared for Sarasota Bay Estuary Program 111 S. Orange Avenue, Suite 200W Sarasota, Florida 34236 June 4, 2012 TABLE OF CONTENTS LIST OF FIGURES ........................................................................................................................................ iii LIST OF TABLES .......................................................................................................................................... v ACKNOWLEDGEMENTS ............................................................................................................................ vii SUMMARY .................................................................................................................................................... ix INTRODUCTION ........................................................................................................................................... 1 METHODS .................................................................................................................................................... 2 Study Area ............................................................................................................................................... -

Prp180---2017-Nelson-Et-Al.Pdf
&RPSDUDWLYH%LRFKHPLVWU\DQG3K\VLRORJ\3DUW& ² Contents lists available at ScienceDirect Comparative Biochemistry and Physiology, Part C journal homepage: www.elsevier.com/locate/cbpc Cardio-respiratory function during exercise in the cobia, Rachycentron 0$5. canadum: The impact of crude oil exposure Derek Nelsona, John D. Stieglitzb, Georgina K. Coxb, Rachael M. Heuera, Daniel D. Benettib, Martin Grosellb, Dane A. Crossley IIa,⁎ a University of North Texas, Department of Biological Sciences, 1155 Union Circle, Denton, TX 76203, United States b Division of Marine Biology and Ecology, Rosenstiel School of Marine and Atmospheric Sciences, University of Miami, Miami, FL 33149-1098, United States ABSTRACT Aerobic exercise capacity is dependent on the cardiorespiratory system's ability to supply oxygen at a rate that meets energetic demands. In teleost fish crude oil exposure, with the associated polycyclic aromatic hydro- carbons (PAH's), reduces exercise performance and this has been hypothesized to be due to compromised car- diovascular function. In this study, we test this hypothesis by simultaneously measuring cardiovascular per- formance, oxygen consumption, and swim performance in a pelagic teleost, the cobia (Rachycentron canadum). Metabolic rate increased over 300% in both groups during the swim trial but as the fish approached the critical fi swim speed (Ucrit) MO2 was 12% lower in the oil exposed sh. Further, stroke volume was initially 35% lower while heart rate was 15% higher in the oil exposed compared to control fish. Our findings suggested, while aspects of cardiovascular and metabolic function are altered by oil exposure, additional studies are needed to further understand the homeostatic mechanisms that may sustain cardiovascular function at higher exercise intensities in cobia. -

Cobia Database Articles Final Revision 2.0, 2-1-2017
Revision 2.0 (2/1/2017) University of Miami Article TITLE DESCRIPTION AUTHORS SOURCE YEAR TOPICS Number Habitat 1 Gasterosteus canadus Linné [Latin] [No Abstract Available - First known description of cobia morphology in Carolina habitat by D. Garden.] Linnaeus, C. Systema Naturæ, ed. 12, vol. 1, 491 1766 Wild (Atlantic/Pacific) Ichthyologie, vol. 10, Iconibus ex 2 Scomber niger Bloch [No Abstract Available - Description and alternative nomenclature of cobia.] Bloch, M. E. 1793 Wild (Atlantic/Pacific) illustratum. Berlin. p . 48 The Fisheries and Fishery Industries of the Under this head was to be carried on the study of the useful aquatic animals and plants of the country, as well as of seals, whales, tmtles, fishes, lobsters, crabs, oysters, clams, etc., sponges, and marine plants aml inorganic products of U.S. Commission on Fisheries, Washington, 3 United States. Section 1: Natural history of Goode, G.B. 1884 Wild (Atlantic/Pacific) the sea with reference to (A) geographical distribution, (B) size, (C) abundance, (D) migrations and movements, (E) food and rate of growth, (F) mode of reproduction, (G) economic value and uses. D.C., 895 p. useful aquatic animals Notes on the occurrence of a young crab- Proceedings of the U.S. National Museum 4 eater (Elecate canada), from the lower [No Abstract Available - A description of cobia in the lower Hudson Eiver.] Fisher, A.K. 1891 Wild (Atlantic/Pacific) 13, 195 Hudson Valley, New York The nomenclature of Rachicentron or Proceedings of the U.S. National Museum Habitat 5 Elacate, a genus of acanthopterygian The universally accepted name Elucate must unfortunately be supplanted by one entirely unknown to fame, overlooked by all naturalists, and found in no nomenclator. -

Species Profile: Pigfish, Orthopristis Chrysoptera
Southern regional SRAC Publication No. 7209 aquaculture center October 2011 VI PR Species Profile: Pigfish, Orthopristis chrysoptera Cortney L. Ohs,1 Matthew A. DiMaggio,1 Scott W. Grabe1 The pigfish,Orthopristis chrysoptera (Fig. 1), is a member of the grunt family, Haemulidae. Haemulids comprise 17 genera and as many as 150 species. Haemulids are distributed throughout the Atlantic, Indian, and Pacific Oceans and are mostly marine, although some brackish and freshwater species exist. Haemulids are called grunts because they can make a grunting or chatter- ing noise by rubbing their pharyngeal teeth together. Pigfish are distinguished from other Figure 1. Adult pigfish, Orthopristis chrysoptera. grunt species throughout their range by several key morphological differences. The dorsal fin of pigfish usually has 12 to 13 spines followed handling, are euryhaline, tolerate high densities, repro- by 15 to 16 soft rays, while the anal fin has three spines duce in tanks, grow rapidly, have established markets with 12 to 13 soft rays. Both the dorsal and anal fin spines with high demand, and are marketed as a baitfish. are covered by a deep, scaly sheath, unlike the soft rays. There are 53 to 58 pored, lateral-line scales in ten longitu- Geographic distribution and habitat dinal rows above the lateral line and 15 to 19 rows below. The chin has a median groove. The ovate-elliptical body Pigfish occur in the Gulf of Mexico from Florida to is considerably compressed, resulting in a body depth the Yucatan peninsula and along the Atlantic coast of the that is 30 to 38 percent of their standard length (SL). -

Andrea RAZ-GUZMÁN1*, Leticia HUIDOBRO2, and Virginia PADILLA3
ACTA ICHTHYOLOGICA ET PISCATORIA (2018) 48 (4): 341–362 DOI: 10.3750/AIEP/02451 AN UPDATED CHECKLIST AND CHARACTERISATION OF THE ICHTHYOFAUNA (ELASMOBRANCHII AND ACTINOPTERYGII) OF THE LAGUNA DE TAMIAHUA, VERACRUZ, MEXICO Andrea RAZ-GUZMÁN1*, Leticia HUIDOBRO2, and Virginia PADILLA3 1 Posgrado en Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, Ciudad de México 2 Instituto Nacional de Pesca y Acuacultura, SAGARPA, Ciudad de México 3 Facultad de Ciencias, Universidad Nacional Autónoma de México, Ciudad de México Raz-Guzmán A., Huidobro L., Padilla V. 2018. An updated checklist and characterisation of the ichthyofauna (Elasmobranchii and Actinopterygii) of the Laguna de Tamiahua, Veracruz, Mexico. Acta Ichthyol. Piscat. 48 (4): 341–362. Background. Laguna de Tamiahua is ecologically and economically important as a nursery area that favours the recruitment of species that sustain traditional fisheries. It has been studied previously, though not throughout its whole area, and considering the variety of habitats that sustain these fisheries, as well as an increase in population growth that impacts the system. The objectives of this study were to present an updated list of fish species, data on special status, new records, commercial importance, dominance, density, ecotic position, and the spatial and temporal distribution of species in the lagoon, together with a comparison of Tamiahua with 14 other Gulf of Mexico lagoons. Materials and methods. Fish were collected in August and December 1996 with a Renfro beam net and an otter trawl from different habitats throughout the lagoon. The species were identified, classified in relation to special status, new records, commercial importance, density, dominance, ecotic position, and spatial distribution patterns. -

Redalyc.Isopods (Isopoda: Aegidae, Cymothoidae, Gnathiidae)
Revista de Biología Tropical ISSN: 0034-7744 [email protected] Universidad de Costa Rica Costa Rica Bunkley-Williams, Lucy; Williams, Jr., Ernest H.; Bashirullah, Abul K.M. Isopods (Isopoda: Aegidae, Cymothoidae, Gnathiidae) associated with Venezuelan marine fishes (Elasmobranchii, Actinopterygii) Revista de Biología Tropical, vol. 54, núm. 3, diciembre, 2006, pp. 175-188 Universidad de Costa Rica San Pedro de Montes de Oca, Costa Rica Available in: http://www.redalyc.org/articulo.oa?id=44920193024 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Isopods (Isopoda: Aegidae, Cymothoidae, Gnathiidae) associated with Venezuelan marine fishes (Elasmobranchii, Actinopterygii) Lucy Bunkley-Williams,1 Ernest H. Williams, Jr.2 & Abul K.M. Bashirullah3 1 Caribbean Aquatic Animal Health Project, Department of Biology, University of Puerto Rico, P.O. Box 9012, Mayagüez, PR 00861, USA; [email protected] 2 Department of Marine Sciences, University of Puerto Rico, P.O. Box 908, Lajas, Puerto Rico 00667, USA; ewil- [email protected] 3 Instituto Oceanografico de Venezuela, Universidad de Oriente, Cumaná, Venezuela. Author for Correspondence: LBW, address as above. Telephone: 1 (787) 832-4040 x 3900 or 265-3837 (Administrative Office), x 3936, 3937 (Research Labs), x 3929 (Office); Fax: 1-787-834-3673; [email protected] Received 01-VI-2006. Corrected 02-X-2006. Accepted 13-X-2006. Abstract: The parasitic isopod fauna of fishes in the southern Caribbean is poorly known. In examinations of 12 639 specimens of 187 species of Venezuelan fishes, the authors found 10 species in three families of isopods (Gnathiids, Gnathia spp. -
Zootaxa, New Record of the Smalleye Stingray, Dasyatis Microps
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/235659724 New record of the smalleye stingray, Dasyatis microps (Myliobatiformes: Dasyatidae), from the western Indian Ocean Article in Zootaxa · March 2008 DOI: 10.11646/zootaxa.1734.1.5 · Source: OAI CITATIONS READS 8 551 3 authors: Simon James Pierce William Toby White Marine Megafauna Foundation CSIRO Oceans & Atmosphere Flagship 117 PUBLICATIONS 1,919 CITATIONS 251 PUBLICATIONS 6,199 CITATIONS SEE PROFILE SEE PROFILE Andrea Denise Marshall Marine Megafauna Foundation 138 PUBLICATIONS 2,456 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Madagascar Whale Shark Project View project The Chondrichthyan Tree of Life Project View project All content following this page was uploaded by Simon James Pierce on 21 May 2014. The user has requested enhancement of the downloaded file. TERM OF USE This pdf is provided by Magnolia Press for private/research use. Commercial sale or deposition in a public library or website site is prohibited. Zootaxa 1734: 65–68 (2008) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Correspondence ZOOTAXA Copyright © 2008 · Magnolia Press ISSN 1175-5334 (online edition) New record of the smalleye stingray, Dasyatis microps (Myliobatiformes: Dasyatidae), from the western Indian Ocean SIMON J. PIERCE1,2,4, WILLIAM T. WHITE3 & ANDREA D. MARSHALL1,2 1Manta Ray & Whale Shark Research Centre, Tofo Beach, Mozambique 2School of Biomedical Sciences, The University of Queensland, St Lucia, QLD 4072, Australia 3CSIRO Marine & Atmospheric Research, GPO Box 1538, Hobart, TAS 7001, Australia 4Corresponding author. E-mail: [email protected] Members of the Dasyatidae (stingrays) range in width from very small (<24 cm, e.g. -

Sharkcam Fishes
SharkCam Fishes A Guide to Nekton at Frying Pan Tower By Erin J. Burge, Christopher E. O’Brien, and jon-newbie 1 Table of Contents Identification Images Species Profiles Additional Info Index Trevor Mendelow, designer of SharkCam, on August 31, 2014, the day of the original SharkCam installation. SharkCam Fishes. A Guide to Nekton at Frying Pan Tower. 5th edition by Erin J. Burge, Christopher E. O’Brien, and jon-newbie is licensed under the Creative Commons Attribution-Noncommercial 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc/4.0/. For questions related to this guide or its usage contact Erin Burge. The suggested citation for this guide is: Burge EJ, CE O’Brien and jon-newbie. 2020. SharkCam Fishes. A Guide to Nekton at Frying Pan Tower. 5th edition. Los Angeles: Explore.org Ocean Frontiers. 201 pp. Available online http://explore.org/live-cams/player/shark-cam. Guide version 5.0. 24 February 2020. 2 Table of Contents Identification Images Species Profiles Additional Info Index TABLE OF CONTENTS SILVERY FISHES (23) ........................... 47 African Pompano ......................................... 48 FOREWORD AND INTRODUCTION .............. 6 Crevalle Jack ................................................. 49 IDENTIFICATION IMAGES ...................... 10 Permit .......................................................... 50 Sharks and Rays ........................................ 10 Almaco Jack ................................................. 51 Illustrations of SharkCam -

Feeding Habits of Centropomus Undecimalis (Actinopterygii, Centropomidae) in the Parnaíba River Delta, Piauí, Brazil
Brazilian Journal of Development 39536 ISSN: 2525-8761 Feeding habits of Centropomus undecimalis (Actinopterygii, Centropomidae) in the Parnaíba river delta, Piauí, Brazil Alimentação do Centropomus undecimalis (Actinopterygii, Centropomidae) no estuário do delta do rio Parnaíba, Piauí, Brasil DOI:10.34117/bjdv7n4-423 Recebimento dos originais: 07/03/2021 Aceitação para publicação: 16/04/2021 José Rafael Soares Fonseca Doutorando em Recursos Pesqueiros e Engenharia de Pesca Programa de Pós-Graduação em Recursos Pesqueiros e Engenharia de Pesca, Centro de Engenharias e Ciências Exatas, Universidade Estadual do Oeste do Paraná – UNIOESTE, Rua da Faculdade, 645, 85903-000 – Toledo– PR – Brasil E-mail: [email protected] Cezar Augusto Freire Fernandes Doutorado em Recursos Pesqueiros e Aquicultura Universidade Federal do Delta do Parnaíba – UFDPAR, Av. São Sebastião, 2819 Bairro Nossa Senhora de Fátima– CEP: 64.202-020 – Parnaíba – PI – Brasil E-mail: [email protected] Francisca Edna de Andrade Cunha Doutorado em Ciências Biológicas Universidade Federal do Delta do Parnaíba – UFDPAR, Av. São Sebastião, 2819 Bairro Nossa Senhora de Fátima– CEP: 64.202-020 – Parnaíba – PI – Brasil E-mail: [email protected] ABSTRACT The objective of this work was to evaluate the feeding of Centropomus undecimalis in the estuary of the Parnaíba river delta, with emphasis on diet composition during seasonal variations between dry and rainy seasons. The samples were obtained from artisanal fishing with gillnets, from June 2014 - July 2015. The individuals were measured, weighed and dissected to remove the stomachs. The fish diet was analyzed using the methods: Gravimetric, Frequency of Occurrence, Dominance of the item and Food Index. -

Growth and Mortality of Lagodon Rhomboides (Pisces: Sparidae)
GROWTH AND MORTALITY OF LAGODON RHOMBOIDES (PISCES: SPARIDAE) IN A TROPICAL COASTAL LAGOON IN NORTHWESTERN YUCATAN, MEXICO CRECIMIENTO Y MORTALIDAD DE LAGODON RHOMBOIDES (PISCES: SPARIDAE) EN UNA LAGUNA TROPICAL COSTERA EN EL NOROESTE DE YUCATÁN, MÉXICO José Luis Bonilla-Gómez1*, Jorge A. López-Rocha2, Maribel Badillo Alemán2, Juani Tzeek Tuz2 and Xavier Chiappa-Carrara2 ABSTRACT Growth and mortality were estimated for the Lagodon rhomboides pinfish inhabiting La Carbonera, a tropical coastal lagoon on the northwestern coast of the Yucatan Peninsula, Mexico. A total of 448 juvenile and adult individuals were collected monthly between April 2009 and May 2010. The length-weight relationship was calculated and the monthly variation in the condition factor was analyzed. Growth was estimated through the von Bertalanffy growth equation using a length frequency analysis. In addition, mortality was estimated and analyzed. Results showed that fish caught were between 2.1 and 20.0 cm long with an average length of 9.42 cm. The length-weight relationship showed isometric growth. The von Bertalanffy growth model parameters -1 were: L∞ = 21.0 cm, W∞ = 163.46 g, k = 1.1 year and t0 = - 0.158 years. Instantaneous mortality rates were 2.11 and 2.61 year-1 as estimated by the method used. According to the results, growth estimates of L. rhomboi- des along the northwestern coast of Yucatan are higher than those found in the population studied in Florida, suggesting a strong influence of environmental conditions in the growth pattern of this species. This study provides the first growth and mortality estimates forL. rhomboides in the Yucatan Peninsula, which is relevant for the proper implementation of conservation measures for this species. -
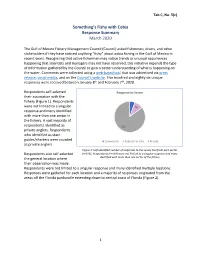
Something's Fishy with Cobia Document
Tab C, No. 5(c) Something’s Fishy with Cobia Response Summary March 2020 The Gulf of Mexico Fishery Management Council (Council) asked fishermen, divers, and other stakeholders if they have noticed anything “fishy” about cobia fishing in the Gulf of Mexico in recent years. Recognizing that active fishermen may notice trends or unusual occurrences happening that scientists and managers may not have observed, this initiative expands the type of information gathered by the Council to gain a better understanding of what is happening on the water. Comments were collected using a web‐based tool that was advertised via press release, social media, and on the Council’s website. Five hundred and eighty‐six unique responses were received between January 8th and February 7th, 2020. Respondents self‐selected Response by Sector their association with the fishery (Figure 1). Respondents 35 were not limited to a singular 60 response and many identified with more than one sector in the fishery. A vast majority of respondents identified as 551 private anglers. Respondents who identified as state guides/charters were counted Commercial Federal For‐hire Private as private anglers. Figure 1: Self‐identified number of responses to the survey tool from each sector Respondents also self‐selected (n=646). Respondents (n=584) were not limited to a singular response and many the general location where identified with more than one sector of the fishery their observation was made. Respondents were not limited to a singular response and many identified multiple locations. Responses were gathered for each location and a majority of responses originated from the areas off the Florida panhandle extending down to central coast of Florida (Figure 2).