Citigroup Jefferies SVB Leerink
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download IPO Report
Donnelley Financial Solutions US IPO Report - December 2020 Edition Congratulations to the 74 December issuers and their advisors on the ~$159 Billion "2020 - an unforgettable year for IPOs! successful completion of their IPO. As I look back on all the successes we Total Raised in 2020 DFIN was proud to have serviced shared with our clients - perseverance, AbCellera Biologics, Altitude hard work, compassion, and great Acquisition, BioAtla, CBRE partnerships led us to successfully Acquisition, 17 Education & 2020 Priced (count) complete 197 IPOs during a year with Technology Group, 4D Molecular 100 so many challenges. An incredibly Therapeutics, Wish, DoorDash, active pipeline, robust valuations, Far Peak Acquisition, Golden vaccine deployment and newfound Falcon Acquisition, Gores hope brings much optimism for 2021." Holding VI, Marquee Raine CRAIG CLAY, PRESIDENT, DONNELLEY FINANCIAL SOLUTIONS 0 Acquisition, Periphas Capital JAN FEB MAR APR MAY JUNE JULY AUG SEP OCT NOV DE… Partnering, Pharming Group NV, Silverback Therapeutics, Thayer 91 League Table *Top 25 Ventures Acquisition, Upstart Issuer's Counsel Count Holdings, and the largest offering IPOs publicly filed in December Ellenoff Grossman 48 this year Airbnb! bringing total count for 2020 to 621. In Kirkland & Ellis 41 2019, there were 293 total filings and Skadden Arps 38 Latham & Watkins LLP 33 296 in 2018. 156 of 2020 filings are Cooley LLP 30 December Priced (count) pending pricing. Goodwin Procter 27 2017 (15) White & Case 23 Davis Polk 19 Sector Breakdown (non-SPACs) 2018 (17) Loeb & Loeb 16 Wilson Sonsini 16 49.8% Healthcare 2019 (13) Winston & Strawn 15 24.08% Technology Ropes & Gray 14 2020 (74) Weil Gotshal 14 11.42% Consumer 2020 Priced Simpson Thacher 11 7.35% Industrial Graubard Miller 9 Greenberg Traurig 9 7.35% Financial 50% of the priced IPOs in 2020 were Paul Weiss 7 SPACs with a total count for the year Sheppard Mullin 7 WilmerHale 7 248 with a total valuation of ~$76 Healthcare Fenwick & West 6 billion. -
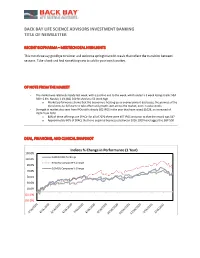
Back Bay Life Science Advisors Investment Banking Title of Newsletter
BACK BAY LIFE SCIENCE ADVISORS INVESTMENT BANKING TITLE OF NEWSLETTER RECENT BIOPHARMA – MEDTECH DEAL HIGHLIGHTS This month we say goodbye to winter and welcome springtime with meals that reflect the transition between seasons. Take a look and find something new to add to your own favorites. OF NOTE FROM THE MARKET - The market was relatively steady last week, with a positive end to the week, which ended a 3 week losing streak: S&P 500 +2.6%; Nasdaq 3.1% (S&) 500 finished at a 52 week high o Market performance shows that the economy is heating up as unemployment decreases, the promise of the discal stimulus bill starts to take effect and growth seen across the market, even in value stocks - Strength in market also seen from IPOs with already 302 IPOS in the year that have raised $102B, an increased of 763% from 2020 o 80% of these offerings are SPACs- for all of 2020 there were 457 IPOS and prior to that the record was 547 o Approximately 60% of SPACs that have acquired businesses between 2016-2020 have lagged the S&P 500 DEAL, FINANCING, AND CLINICAL SNAPSHOT Indices % Change in Performance (1 Year) 130.0% NASDAQ NBI % Change 110.0% NASDAQ Composite % Change 90.0% DJSMDQ Composite % Change 70.0% 50.0% 30.0% 10.0% (10.0%) (30.0%) 3/16/2020 4/16/2020 5/16/2020 6/16/2020 7/16/2020 8/16/2020 9/16/2020 1/16/2021 2/16/2021 10/16/2020 11/16/2020 12/16/2020 As Indices % Change in Performance (1 Month) 120.0% 100.0% 80.0% 60.0% 40.0% NASDAQ NBI % Change 20.0% NASDAQ Composite % Change 0.0% DJSMDQ Composite % Change 3/2/2021 3/5/2021 3/8/2021 2/12/2021 2/15/2021 2/18/2021 2/21/2021 2/24/2021 2/27/2021 3/11/2021 BIOPHARMA-MEDTECH FINANCINGS OF NOTE RECENT BIOPHARMA FINANCINGS Bookrunners / Lead + New Amount Company Description Deal Type Investors Raised ($M) Proceeds will be used to effect a business Frontier combination with one or more businesses in the SPAC Credit Suisse, Berenberg $200 Acquisition biotech space. -

1Life Healthcare, Inc. (Q1 2021 Earnings)
1Life Healthcare, Inc. (Q1 2021 Earnings) May 12, 2021 Corporate Speakers: Rose Salzwedel; 1Life Healthcare, Inc.; Director of IR Amir Rubin; 1Life Healthcare, Inc.; Chair, CEO & President Bjorn Thaler; 1Life Healthcare, Inc.; CFO Participants: Ryan Daniels; William Blair & Company L.L.C.; Partner & Co-Group Head of Healthcare Technology and Services Lisa Gill; JPMorgan Chase & Co; MD, Head of U.S. Healthcare Technology & Distribution Equity Research and Senior Research Analyst Elizabeth Anderson; Evercore ISI Institutional Equities; Associate George Hill; Deutsche Bank AG; MD & Equity Research Analyst Stephanie Wissink; Jefferies LLC; Equity Analyst and MD Alexander Draper; Truist Securities, Inc.; MD of Equity Research Connor Oleferchik; Morgan Stanley; Research Associate Richard Close; Canaccord Genuity Corp.; MD & Senior Analyst Daniel Grosslight; Citigroup Inc.; Research Analyst Stephanie Davis; SVB Leerink LLC; MD of Healthcare Technology and Distribution Jessica Tassan; Piper Sandler & Co.; Research Analyst Carlos Consuegra; Credit Suisse AG; Research Analyst PRESENTATION Operator: Ladies and gentlemen, thank you for standing by, and welcome to the One Medical First Quarter 2021 Earnings Conference Call. (Operator Instructions) I would now like to hand the conference over to your speaker for today, Rose Salzwedel, Head of Investor Relations. You may begin. Rose Salzwedel: Thank you, operator. Hello, everyone, and welcome to One Medical Fiscal 2021 First Quarter Earnings Call. I am joined today by Amir Dan Rubin, Chair and CEO of One Medical; and Bjorn Thaler, Chief Financial Officer of One Medical. A complete disclosure of our results can be found in our press release issued earlier today as well as in our related Form 8-K, all of which are available on our website at investor.onemedical.com. -

$150000000 Bofa Securities Jefferies SVB Leerink
Table of Contents Filed Pursuant to Rule 424(b)(5) Registration No. 333-227182 The information contained in this preliminary prospectus supplement and the accompanying prospectus is not complete and may be changed. This preliminary prospectus supplement and the accompanying prospectus do not constitute an offer to sell these securities and we are not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted. SUBJECT TO COMPLETION, DATED FEBRUARY 10, 2021 PRELIMINARY PROSPECTUS SUPPLEMENT (To Prospectus dated September 4, 2018) $150,000,000 Common Stock We are offering $150,000,000 of shares of our common stock. Our common stock is listed on The Nasdaq Global Market under the symbol “TVTX.” On February 9, 2021, the last reported sale price of our common stock on The Nasdaq Global Market was $30.05 per share. Based on an assumed public offering price of $30.05 per share, the last reported sale price of our common stock on The Nasdaq Global Market on February 9, 2021, we would expect to offer approximately 4,991,680 shares hereby. Investing in our common stock involves risks. See “Risk Factors” on page S-7 of this prospectus supplement and in the documents incorporated by reference into this prospectus supplement. PER SHARE TOTAL Public offering price $ $ Underwriting discounts and commissions (1) $ $ Proceeds to Travere (before expenses) $ $ (1) See “Underwriting” for additional disclosure regarding underwriting compensation. We have granted the underwriters an option for a period of 30 days to purchase up to an additional $22,500,000 of common stock on the same terms set forth above. -

EDITED TRANSCRIPT MRK.N - Q2 2021 Merck & Co Inc Earnings Call
REFINITIV STREETEVENTS EDITED TRANSCRIPT MRK.N - Q2 2021 Merck & Co Inc Earnings Call EVENT DATE/TIME: JULY 29, 2021 / 12:00PM GMT OVERVIEW: Co. reported 2Q21 total Co. revenues of $11.4b and non-GAAP EPS of $1.31. Expects 2021 revenues to be $46.4-47.4b and non-GAAP EPS to be $5.47-5.57. REFINITIV STREETEVENTS | www.refinitiv.com | Contact Us ©2021 Refinitiv. All rights reserved. Republication or redistribution of Refinitiv content, including by framing or similar means, is prohibited without the prior written consent of Refinitiv. 'Refinitiv' and the Refinitiv logo are registered trademarks of Refinitiv and its affiliated companies. JULY 29, 2021 / 12:00PM, MRK.N - Q2 2021 Merck & Co Inc Earnings Call CORPORATE PARTICIPANTS Caroline Litchfield Merck & Co., Inc. - Executive VP & CFO Dean Y. Li Merck & Co., Inc. - EVP Franklin K. Clyburn Merck & Co., Inc. - Executive VP & President Human Health Peter Dannenbaum Merck & Co., Inc. - VP of IR Robert M. Davis Merck & Co., Inc. - President, CEO & Director CONFERENCE CALL PARTICIPANTS Aaron Gal Sanford C. Bernstein & Co., LLC., Research Division - Senior Research Analyst Andrew Simon Baum Citigroup Inc., Research Division - Global Head of Healthcare Research and MD Carter Lewis Gould Barclays Bank PLC, Research Division - Senior Analyst Christopher Thomas Schott JPMorgan Chase & Co, Research Division - Senior Analyst Daina Michelle Graybosch SVB Leerink LLC, Research Division - MD of Immuno-Oncology & Senior Research Analyst Geoffrey Christopher Meacham BofA Securities, Research Division - Research Analyst Louise Alesandra Chen Cantor Fitzgerald & Co., Research Division - Senior Research Analyst & MD Mara Goldstein Mizuho Securities USA LLC, Research Division - MD of Equity Research Department Stephen Michael Scala Cowen and Company, LLC, Research Division - MD & Senior Research Analyst Terence C. -

Global Equity Capital Markets Review
Global Equity Capital Markets Review FIRST NINE MONTHS 2019 | MANAGING UNDERWRITERS Global Equity Capital Markets Review First Nine Months | Managing Underwriters Global Deals Intelligence Global Initial Public Offerings GLOBAL EQUITY CAPITAL MARKETS ACTIVITY HITS SEVEN-YEAR LOW $200 1,400 Equity capital markets activity totaled US$466.0 billion during the first nine months of 2019, an 18% $180 decline compared to a year ago and the slowest first nine months for global equity capital markets 1,200 activity since 2012. By number of issues, 3,199 ECM offerings were brought to market during the first $160 nine months of the year, an 18% decrease compared to a year ago. Compared to the second quarter of this year, third quarter 2019 ECM volume decreased 17%, marking the slowest third quarter for global $140 1,000 equity capital markets activity since 2015. $120 GLOBAL IPOs FALL 27% TO THREE-YEAR LOW; THIRD QUARTER IPO PROCEEDS FALL 39% 800 Global initial public offering activity during the first nine months of 2019 totaled US$105.7 billion, a 27% $100 decrease compared to last year and the slowest opening period for global IPOs since 2016. IPOs on US 600 exchanges fell 61% during the third quarter of 2019, pulling first nine months 2019 proceeds to US$42.2 $80 billion, a 2% decrease compared to a year ago. IPO activity in EMEA decreased 43% compared to a year ago, while Asia Pacific IPO activity fell 34%. During the third quarter of 2019, global IPO activity declined $60 400 39%, by proceeds, and 21%, by number of deals, compared to the second quarter of this year. -

Placementtracker Publishes Q2 2020 PIPE and Private Placement Markets League Tables
PlacementTracker Publishes Q2 2020 PIPE and Private Placement Markets League Tables PlacementTracker, the leading source for data and analysis to institutions in the PIPE and Private Placement markets, today announced the most active investment banks, investors, and law firms in the U.S. PIPE and Private Placement markets for Q2 2020. A total of $42.9 billion was raised in 738 transactions throughout Q2 of 2020. Of this amount, 2 transactions totaling $65 million have been announced but not yet closed as of 07/20/2020. Deal volume has increased 44% (511 deals in 2019) and dollar volume increased 71% ($25.2 billion in 2019). We congratulate all the active members of the private placement community, many of whom are long- time clients of PlacementTracker. We are dedicated to providing access to the very best data and analytics available in our industry. Most Active Participants Include: • H.C. Wainwright & Co., LLC as the most active placement agent • Lincoln Park Capital as the most active institutional investor • Cooley LLP as the most active issuer counsel • Schulte Roth & Zabel, LLP as the most active investor counsel • Ellenoff Grossman & Schole LLP as the most active placement agent counsel • Jefferies & Company, Inc. as most active Sales Agent for At-the-Market (ATM) transactions The Q2 2020 U.S. PIPE and Private Placement Markets Investment Banking League Table by Number of Transactions: Placement Agent Name Transaction Count Total Amount Placed H.C. Wainwright & Co., LLC 114 $3,213,534,642 Maxim Group LLC 47 $492,317,832 Cowen and Company, LLC 28 $3,142,499,808 A.G.P./Alliance Global Partners 27 $332,192,843 Jefferies & Company, Inc. -
Torreya Overview
Torreya Overview February 2020 Securities offered in the United States are offered through Torreya Capital LLC, Member FINRA/SIPC. In Europe such services are offered through Torreya Partners (Europe) LLP, which is authorized and regulated by the UK Financial Conduct Authority. Torreya is a Global Healthcare Investment Banking Firm We are known for: Representative Transaction Work • Deep Relationships Mergers & Acquisitions Acquisition of Sale to Option to be acquired Sale of Majority We have strong personal generics business of Sale to by Stake to relationships across the pharmaceutical and healthcare sectors. $1.05 billion $658 million $215 million June 2017 June 2014 November 2019 January 2019 January 2020 • Operating Perspective Generic Pharma Specialty Pharma Bioproduction Specialty Pharma Biotech Many of our senior Licensing, colleagues come from Asset Sales Sale of industry and bring decades and JVs Sale of FXR program to Intrathecal business to License of NOV03 in Navicixizumab License North America to to China JV with of experience.. • Deal Excellence Up to $306 million Up to $470 million $203 million $50 Million Torreya is known as a firm January 2015 March 2017 December 2019 January 2020 December 2018 that gets tough deals done. Asset Sale / Biotech Divestiture / Sp Pharm License / Biotech License / Biotech JV / Specialty Pharma Our team is skilled in highly Debt, Royalty structured transactions. Sales and PE Royalty-backed Recapitalization and Buy acquisition financing • Recaps Sale of Majority Stake to Credit Facility from Sale of Majority Stake to Healthcare Focus of Nextwave from for undisclosed Our healthcare focus spans spec pharma pharma, biotech, $125 million $125 million $70 million $100 million bioproduction, pharma April 2019 September 2018 October 2019 February 2019 February 2016 services, physician services PE Recap / Generics Debt / Spec Pharma Debt / Services PE Recap / CRO Royalty / Spec Pharm and HCIT. -

4500000 Shares Bofa Securities Jefferies SVB Leerink
Table of Contents The information in this preliminary prospectus supplement and the accompanying prospectus, relating to an effective registration statement under the Securities Act of 1933, as amended, is not complete and may be changed. A registration statement relating to these securities has been declared effective by the Securities and Exchange Commission. This preliminary prospectus supplement and the accompanying prospectus are not an offer to sell these securities and we are not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.. Filed Pursuant to Rule 424(b)(5) Registration No. 333-229499 SUBJECT TO COMPLETION, DATED SEPTEMBER 21, 2020 PRELIMINARY PROSPECTUS SUPPLEMENT (To Prospectus dated February 1, 2019) 4,500,000 Shares Common Stock We are offering 4,500,000 shares of our common stock. Our common stock is listed on the Nasdaq Global Market under the symbol “ACET.” On September 18, 2020, the last reported sale price of our common stock was $21.40 per share. We are an “emerging growth company” as defined by the Jumpstart Our Business Startups Act of 2012 and, as such, we have elected to comply with certain reduced public company reporting requirements for this prospectus supplement, the accompanying prospectus and future filings. Investing in our common stock involves a high degree of risk. Please read “Risk Factors ” beginning on page S-10 of this prospectus supplement and in the documents incorporated by reference into this prospectus supplement. Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement or accompanying prospectus is truthful or complete. -

TRANSCRIPT MYOV – Q2 2020 Myovant Sciences, Inc
EDITED TRANSCRIPT MYOV – Q2 2020 Myovant Sciences, Inc. Earnings Call EVENT DATE / TIME: NOVEMBER 12, 2020 / 8:30 AM ET OVERVIEW: Co. reported second fiscal quarter 2020 financial results and provided a general business update. NOVEMBER 12, 2020 / 8:30 AM ET, MYOV – Q2 2020 Myovant Sciences, Inc. Earnings Call CORPORATE PARTICIPANTS Ryan Crowe; Myovant Sciences; Vice President of Investor Relations Lynn Seely; Myovant Sciences; CEO Adele Gulfo; Myovant Sciences; Interim Chief Commercial Officer Frank Frabe; Myovant Sciences; President & CFO Juan Camilo Arjona; Myovant Sciences; Chief Medical Officer CONFERENCE CALL PARTICIPANTS Jason Butler; JMP Securities LLC; Analyst Ami Fadia; SVB Leerink; Analyst James Shin; Citigroup Inc.; Analyst Eric Joseph; JPMorgan Chase & Co.; Analyst Brian Skorney; Robert W. Baird & Co.; Analyst Phil Nadeau; Cowen and Company, LLC; Analyst Paul Choi; Goldman Sachs Group Inc.; Analyst PRESENTATION Operator Good day, everyone and welcome to Myovant Sciences Second Fiscal Quarter 2020 Earnings Conference Call. Today's call is being recorded. At this time, I would like to turn the call over to Mr. Ryan Crowe, Vice President of Investor Relations at Myovant. Please go ahead. __________________________________________________________________________________ Ryan Crowe – Myovant Sciences, Inc. – VP of IR Thank you, Operator. Good morning and thanks for joining us today for a general business update and to review Myovant's second fiscal quarter 2020 financial results. Joining me for today's call are Dr. Lynn Seely, Myovant's Chief Executive Officer; Frank Karbe, President and Chief Financial Officer; Adele Gulfo, Interim Chief Commercial Officer; and Dr. Juan Camilo Arjona, Chief Medical Officer. In addition to the press release issued earlier this morning, the slides that will be presented during today's webcast are available on our Investor Relations website, investors.myovant.com. -

Q4 2020 Pitchbook/NVCA Venture Monitor
Q4 2020 In partnership with Annual VC investment tops Massive IPOs drive record year Established fund managers $150 billion for first time ever for VC exits find success during pandemic Page 5 Page 29 to push fundraising to new highs Page 31 The definitive review of the US venture capital ecosystem Credits & contact PitchBook Data, Inc. JOHN GABBERT Founder, CEO ADLEY BOWDEN Vice President, Research & Analysis RESEARCH CAMERON STANFILL, CFA Senior Analyst, VC KYLE STANFORD, CAIA Analyst, VC JOSHUA CHAO, Ph.D. Analyst, VC [email protected] DATA ALEX WARFEL Data Analyst Contents Report & cover design by JULIA MIDKIFF and MARA POTTER Executive summary 3 National Venture Capital Association (NVCA) BOBBY FRANKLIN President & CEO NVCA policy highlights 4 MARYAM HAQUE Senior Vice President of Industry Advancement Overview 5 CASSIE ANN KIGGEN Director of Communications DEVIN MILLER Manager of Communications & Digital Angel, seed, and first financings 7 Strategy MICHAEL CHOW Research Director, NVCA and Early-stage VC 9 Venture Forward Late-stage VC 11 Contact NVCA nvca.org Regional spotlight 15 [email protected] Deals by sector 16 Venture Forward MARYAM HAQUE Executive Director SVB: Why SPACs now? 20 Silicon Valley Bank Female founders 22 GREG BECKER Chief Executive Officer MICHAEL DESCHENEAUX President Nontraditional investors 24 DEVIKA PATIL Managing Director of Venture Capital Velocity Global: Slow the cash burn for portco global Relationships 26 SUSAN WINTER Head of Syndications expansion Contact Silicon Valley Bank Exits 29 svb.com [email protected] Fundraising 31 Q4 2020 league tables 33 Velocity Global Methodology 35 BEN WRIGHT Founder and Chief Executive Officer ROB WELLNER Chief Revenue Officer ANNIE THOMPSON President of Strategic Partnerships Contact Velocity Global velocityglobal.com [email protected] 2 Q4 2020 PITCHBOOK-NVCA VENTURE MONITOR Executive summary Despite facing macro headwinds from the COVID-19 pandemic for most of 2020, the US VC industry remained resilient on a broad level. -

Fiscal 2019 Third Quarter Conference Call Transcript
FISCAL 2019 THIRD QUARTER CONFERENCE CALL TRANSCRIPT May 7, 2019 / 08:00 AM EDT On May 7, 2019, Premier, Inc. hosted a conference call to discuss financial results for the fiscal 2019 third quarter, ended March 31, 2019. The following transcript is an interpretation of the statements made on the call. The actual conference call may have differed slightly. CORPORATE PARTICIPANTS James R. Storey Premier, Inc. – VP of IR Susan D. DeVore Premier, Inc. – CEO and Director Michael J. Alkire Premier, Inc. – President Craig S. McKasson Premier, Inc. – CFO, CAO, Senior VP & Treasurer CONFERENCE CALL PARTICIPANTS Charles Rhyee Cowen and Company, LLC, Research Division – MD and Senior Research Analyst Chun-Wai Yong Barclays Bank PLC, Research Division – Research Analyst David M. Larsen SVB Leerink LLC, Research Division – MD, Healthcare Information Technology and Distribution Eric R. Percher Nephron Research LLC – Research Analyst Kevin Caliendo UBS Investment Bank, Research Division – Equity Research Analyst of Healthcare IT and Distribution Michael Aaron Cherny BofA Merrill Lynch, Research Division - Director Ryan Scott Daniels William Blair & Company L.L.C., Research Division – Partner and Healthcare Analyst Sean Wilfred Dodge Jefferies LLC, Research Division – Equity Analyst Sean William Wieland Piper Jaffray Companies, Research Division – MD & Senior Research Analyst Stephanie July Demko Citigroup Inc, Research Division – VP & Senior Analyst P R E S E N T A T I O N Operator Good day, ladies and gentlemen, and welcome to the Premier Inc. Fiscal 2019 Third Quarter Results and Conference Call. (Operator Instructions) As a reminder, this conference call is being recorded. I would now like to introduce your host for today's conference, Mr.