Contributors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bankruptcy Forms for Non-Individuals, Is Available
Case 16-50379 Doc 1 Filed 01/05/16 Entered 01/05/16 17:02:40 Desc Main Document Page 1 of 27 Fill in this information to identify your case: United States Bankruptcy Court for the: NORTHERN DISTRICT OF GEORGIA Case number (if known) Chapter you are filing under: Chapter 7 Chapter 11 Chapter 12 Chapter 13 Check if this an amended filing Official Form 201 Voluntary Petition for Non-Individuals Filing for Bankruptcy 12/15 If more space is needed, attach a separate sheet to this form. On the top of any additional pages, write the debtor's name and case number (if known). For more information, a separate document, Instructions for Bankruptcy Forms for Non-Individuals, is available. 1. Debtor's name Titan Team Management, LLC 2. All other names debtor used in the last 8 years Include any assumed names, trade names and doing business as names 3. Debtor's federal Employer Identification 45-3985275 Number (EIN) 4. Debtor's address Principal place of business Mailing address, if different from principal place of business 2150 Boggs Road, Suite 300/370 Duluth, GA 30096 Number, Street, City, State & ZIP Code P.O. Box, Number, Street, City, State & ZIP Code Gwinnett Location of principal assets, if different from principal County place of business Number, Street, City, State & ZIP Code 5. Debtor's website (URL) titanteamsports.com 6. Type of debtor Corporation (including Limited Liability Company (LLC) and Limited Liability Partnership (LLP)) Partnership Other. Specify: Official Form 201 Voluntary Petition for Non-Individuals Filing for Bankruptcy page 1 Case 16-50379 Doc 1 Filed 01/05/16 Entered 01/05/16 17:02:40 Desc Main Document Page 2 of 27 Debtor Titan Team Management, LLC Case number (if known) Name 7. -

Powered by ISA Baseball Teams Elite Instruction Program
Powered By ISA Baseball Teams Elite Instruction Program Welcome to our POWERED by program! ISA instructors provide position specific skill instruction for your established team or program. Our program curriculum is tailored to age, skill and ability level. Our goal is to help each young athlete solidify their mechanical foundation and maximize their individual potential, ultimately creating an unstoppable ball team. ISA is centered in creating a progression-based instructional curriculum, complete with throwing mechanics, hitting, defense, pitching/catching, and game situations/baseball IQ & awareness. ISA instructors are full time baseball professionals who teach players from ages 8 to 18. Each instructor has unique experience, ranging from collegiate to professional and MLB levels. All ages receive 1x week practice time slot (October-March, pending package), with options for a second practice (January- April) for an additional price. Coaches are free to organize and run their own team practice. Open Facility Use All athletes have access to the facility 2:30-4:30 during the school year (excludes holidays). Players can hit, take ground balls and workout. Facility use during these times is unlimited. Partnered Vendors Marucci - Uniforms/Apparel/Equipment Marucci Sports is an official partner of Impact Sports Academy and a top supplier in Major League Baseball. Owned and operated by current and former MLB players, Marucci is endorsed by many professional athletes. Through our partnership, ISA is able to offer an exclusive discount on Marucci products. Each POWERED By team will be provided their own designated Marucci website to order uniform packages, team branded merchandise, baseball equipment and gear with discounts of up to 20% off. -

Mouthguards, Baseball, Shoe Care Versatile Winter Jackets
LICENSING TRENDS GENERATION Z HOT NEW PRODUCTS COOL CUSTOMERS Fashion & Fanatacism The Key Demographic Mouthguards, Baseball, Shoe Care Versatile Winter Jackets N B A P L A Y E R A N D A N D 1 P I T C H M A N L A N C E S T E P H E N S O N FORECASTING THE KEY TRENDS FOR 2015 PERMIT # 191 # PERMIT ITHACA, NY ITHACA, PAID US POSTAGE US RR STD PRSRT JANUARY/FEBRUARY 2015 Executive Editor Mark Sullivan [email protected] 646-319-7878 Editor-in-Chief Cara Griffin [email protected] www.sportsinsightmag.com www.facebook.com/sportsinsight twitter.com/sports_insight Art Director Francis Klaess Associate Art Director Mary McGann Contributors Jennifer Ernst Beaudry Suzanne Blecher Michael Jacobsen Nancy Ruhling Tim Sitek Publisher Jeff Gruenhut JANFEB15 [email protected] “The merging of lifestyle and performance (athleisure) is the trend of today and future seasons.” 404-467-9980 Advertising Fandom and fashion are driving the style trends in sports licensed product for 2015. Page 22 Beth Gordon [email protected] 949-293-1378 Troy Leonard [email protected] 8 30 352-624-1561 Matthew Mauer IN THE MARKET SHOE CARE 516-305-4710 [email protected] Reebok’s UFC Deal; Retail tips for Jeff Nott Lance Stephenson selling insoles and a [email protected] Q&A; Retail variety of footwear 516-305-4711 Insight and more accessories. Sam Selvaggio industry analysis. [email protected] 212-398-5021 Production Angela Zavulunova 516 305-4712 [email protected] 18 34 Subscriptions store.formula4media.com LICENSING BASEBALL Business Manager Vendors weigh in A roundup of the Marianna Rukhvarger with key trends to latest and greatest 516-305-4709 [email protected] watch in the sports new gear in the Promotion Director licensing category baseball world. -

2013 Approved /Licensed Bats
Updated 06/10/2013 2013 Approved /Licensed Bats 2013 DYB Approved/Licensed 2 1/4" Bat Listing The following DYB licensed 2 ¼” diameter bats are approved for the 2013 baseball season in all divisions of Dixie Youth Baseball. Important: This is a list of licensed metal and composite bats, not a list of all possible bats that could be used. Provided the bat meets the specifications of DYB Rule 1.10, it may be used. Any bat, even those appearing on this list, must meet all the specifications of Rule 1.10 for the particular division of play, including specifications regarding length, weight, diameter, markings, etc. Newly manufactured bats will have the following stamped on the barrel Approved for Play in Dixie Youth Baseball, BPF 1.15 and Year (ex. 2012) – Note: T‐Ball bats may be used to hit balls off a stationery tee ONLY. Dixie Youth Baseball reserves the right to add or delete bats on this approved listing. Adidas / Dicks Sporting Goods Barrel Material Handle Material Model Number Model Name Metal Metal YBVQSH2K10 Vanquish Metal Metal YBVQSH2K11 Vanquish Metal Metal YBVQSH2K12 Vanquish Anderson Bat Company Barrel Material Handle Material Model Number Model Name Metal Metal 015012 PyroTech XP Metal Metal 015013 Techzilla Metal Metal 015014 Techzilla XP Metal Metal 015015 KXP Metal Metal 015016 Techzilla XP Metal Metal 015017 NanoTek XP Metal Metal 015019 NanoTek XP Metal Metal 015020 Techzilla XP Metal Metal 015022 Ignite XP -11 Metal Composite 015024 NanoTek XP Metal Composite 015025 NanoTek XP Baden Sports Inc Barrel Material Handle -

Baseball/Softball Batter's Faceguard Legacy Product List
SEI Participant Brand Name Model Model Information Program Baseball/Softball Faceguard for All-Star Batters Helmet Ampac Enterprises Inc All-Star BHFG2-S7-1 NOCSAE: Baseball/Softball Helmet Face Protector (ND072-04) Models BH3000 and BH3010 Baseball/Softball Faceguard for Under Armour Batters Ampac Enterprises Inc Under Armour UABH-FGB2-1 Helmet Models UABH 100, UABH 110, UABH2 100 and NOCSAE: Baseball/Softball Helmet Face Protector (ND072-04) UABH2 150 Baseball/Softball Faceguard for Under Armour Batters Ampac Enterprises Inc Under Armour UABH-FGB Helmet Models UABH-100, UABH2-100, UABH2-150, NOCSAE: Baseball/Softball Helmet Face Protector (ND072-04) UABH-110 and UABH2-110 Baseball/Softball Faceguard forAll-Star Batters Helmet Ampac Enterprises Inc All-Star BHFG-S7-1 NOCSAE: Baseball/Softball Helmet Face Protector (ND072-04) Models BH3000 Baseball/Softball Face Protector, One Size; Fits H4 batter's Champro Sports CHAMPRO H4FM NOCSAE: Baseball/Softball Helmet Face Protector (ND072-04) helmets in sizes Adult, Youth and T-Ball Champro Sports CHAMPRO H4FMS Softball Face Protector, One Size; Fits H4 batter's helmets NOCSAE: Baseball/Softball Helmet Face Protector (ND072-04) For use with DBH-1 batter's helmet sizes: XS (6 - 6-3/4), SM Diamond Sports Diamond Sports BH-1 FG NOCSAE: Baseball/Softball Helmet Face Protector (ND072-04) (6-3/8 - 6-7/8), MED (6-7/8 - 7-1/4), LG (7-1/4 - 7-1/2) Softball Face Protector mounted on Trilogy Fast-Pitch Dick's Sporting Goods Adidas BTE00300 NOCSAE: Baseball/Softball Helmet Face Protector (ND072-04) helmet; -

All Manufacturers Legal Bats
Certification Number Manufacturer Model Length Barrel Classification NCAA-02-0042 Akadema Xtension 32 A NCAA-02-0043 Akadema Xtension 33 A NCAA-02-0044-2 Akadema Xtension & Xtension Catapult 34 A NCAA-03-0114 Akadema Xtension & Xtension Catapult 33 A NCAA-03-0122 Akadema Xtension & Xtension Catapult 32 A NCAA-05-0007 Akadema Xtension & Xtension Catapult 31 A NCAA-07-0160 Akadema Xtension Catapult & Catapult Ice 34 A NCAA-07-0164 Akadema Xtension Catapult & Catapult Ice 33 A NCAA-07-0165 Akadema Xtension Catapult & Catapult Ice 32 A NCAA-08-0022-4 Akadema Xtension Catapult & Catapult Ice 31 A NCAA-05-0124 Anderson Bat 0140013431 & 0140043431 & 0140053431 (PyroTechXS) 34 A NCAA-05-0125-2 Anderson Bat 0140013330 & 0140043330 & 0140053330 (PyroTechXS) 33 A NCAA-05-0126-4 Anderson Bat 0140013229 & 0140043229 & 0140053229 (PyroTechXS) 32 A NCAA-08-0160 Anderson Bat 014006 (NanoTek XS) 34 A NCAA-08-0162-2 Anderson Bat 014006 (NanoTek XS) 32 A NCAA-08-0161-3 Anderson Bat 014006 (NanoTek XS) 33 A D. Same as handle, solid "d" construction NCAA-99-0022 Baum AAA Pro 33.5 unchanged since introduction in 1992 ncaa has details in possession D. Same as handle, solid "d" construction NCAA-99-0023 Baum AAA Pro 34 unchanged since introduction in 1992 ncaa has details in possession Barrel Classification Options: A - Hollow Aluminum B - Solid Wood, Wood Laminate, Bamboo Laminate As of February 15, 2010 1 C - Hollow Fiber Composite - Illegal D - Other (incl. multiple materials) - Describe Construction Certification Number Manufacturer Model Length Barrel Classification D. Same as handle, solid "d" construction NCAA-99-0024 Baum AAA Pro 32 unchanged since introduction in 1992 ncaa has details in possession D. -

Top Trends in Sports Licensing
LINEUP CHANGE SAFETY FIRST KNIT IT UP ON THE RISE Baseball’s Bat Shakeup New Mouthguard Tech Must-Have Sneakers Sports Bra Style TRENDS, PERSPECTIVE AND ANALYSIS BRINGING IT ON HOME TOP TRENDS IN SPORTSINSIGHTMAG.COM SPORTS LICENSING BIG STORIES IMPACTING THE INDUSTRY IN 2017 A FORMULA4 MEDIA PUBLICATION / JANUARY/FEBRUARY 2017 Executive Editor Mark Sullivan [email protected] 646-319-7878 Editor-in-Chief Cara Griffin [email protected] January/February 2017 Senior Editor Bob McGee [email protected] 12 Art Director Francis Klaess KNIT IT UP Associate Art Director The trend du jour is the knit Mary McGann sneaker — now available on Contributors shoe walls everywhere. Jennifer Ernst Beaudry Suzanne Blecher Michael Jacobsen Tim Sitek 14 Publisher STEP UP Jeff Gruenhut [email protected] Emerging technologies 404-467-9980 are changing the game Advertising in the insole market. Christina Henderson 516-305-4710 [email protected] 16 Troy Leonard [email protected] SAFETY FIRST 352-624-1561 New mouthguard technologies Jeff Nott [email protected] and marketing strategies are 516-305-4711 driving the market ahead. Katie O’Donohue [email protected] 828-244-3043 18 Sam Selvaggio BRINGING IT ON HOME [email protected] 212-398-5021 Fashion trends, new Production demographics and a new Brandon Christie world order in sports licensing. 516-305-4710 [email protected] Subscriptions 22 store.formula4media.com ON THE RISE Business Manager Marianna Rukhvarger Innovative R&D combined with 516-305-4709 athleisure trends have the sports [email protected] bra category in the spotlight. 26 LINEUP CHANGE PO Box 23-1318 New baseball bat regulations Great Neck, NY 11023 mean there’s a shakeup for Phone: 516-305-4710 Fax: 516-441-5692 brands and retailers. -

0272 James V Negri 0274 Burnet Hamilton 0276 S Marguerite Astrom
31 6 7 5 6 7 5 6 7 5 6 7 5 6 7 5 0272 James V Negri 0461 Mrs Flora C O'Connell 0634 Herbert R Sill 0838 Paul M Sanderson 1009 Irving J Lehman 0274 Burnet Hamilton 0464 Sheltered Work Room 0635 Kenneth L Hoffman 0839 Gertrude D Dowd 1011 Roger Hinds 0276 S Marguerite Astrom (Essex County of) 0636 Oliver J Hess 0840 Mrs Charlotte R Wilson 1017 Mrs Etta M Wilson 0277 Rev A Junious Edwards 0466 Sherman Hinton 0639 Mrs Lena Whittman 0842 Edmund D Grimes 1019 Mrs Sarah Levine 0279 DiPietro Bros Pork 0467 Mrs Fanny Holzer 0640 Andrew F Dempsey 0844 John C Giuliano 1020 W Frederick Harding Store 0469 Salvatore Russomanno 0642 George F Urna 0845 Michael A Butler 1021 Lillian Hohen 0280 John N Carleo 0470 Charles R Jones 0643 Alfred Spyies 0846 Arthur B Baptiste 1025 John H High 0281 A Canfield & Co 0472 Anthony Cucco 0645 Mrs Hannah Ellis 0847 Leonard Dimmick 1026 American Window 0283 Allen B Planer 0475 Quality Color Laboratory 0648 Charles T Johnson 0848 Lawrence F Bond Shade Co 0284 Dominick M Castoro 0478 Melvin Feiler 0649 Carmel P ladarola 0849 Mitchell Black 1029 Annie Batchelor 0286 John J Lorusso 0479 Leonard N Englebrook 0651 Chris Christensen 0854 Mrs Mary J Ryan i035 A Martin Schmidt 0287 Mrs Agnes Buchanan 0480 John A Rogers 0653 Milton Barnert 0855 Mrs Ruth H Ike 1037 John W Weller Jr 0288 Roy Gamble 0481 Mrs Mary Walker 0658 William A Lamb Jr 0856 George R Leone 1038 Mrs Mary L Boling 0290 Fred Drummond 0483 Joseph J F Leifken 0659 Thatcher J Levens 0857 Mrs Virginia W Fooks 1042 Philip Falcone 0293 Dominick N Giordano 0484 Ethel -

Commencement, 2004 Marshall University
Marshall University Marshall Digital Scholar Marshall University Commencement University Archives 2004 Commencement, 2004 Marshall University Follow this and additional works at: https://mds.marshall.edu/commencement Part of the Higher Education Commons Recommended Citation Marshall University, "Commencement, 2004" (2004). Marshall University Commencement. 28. https://mds.marshall.edu/commencement/28 This Program is brought to you for free and open access by the University Archives at Marshall Digital Scholar. It has been accepted for inclusion in Marshall University Commencement by an authorized administrator of Marshall Digital Scholar. For more information, please contact [email protected], [email protected]. :-: • •.:•....... Commencement 2004 Marshall University The One Hundred S1xty,Seventh Cotnmencetnent Marshall University Alma Mater Marshall Gracious Alma Mater, May the years be kind to Marshall; We thy name revere: May she grow infame; May each noble son and daughter May her children fail her never Cherish thine honor dear. True to her beacon flame. May thy lamp be ever bright May her spirit brave and strong Guiding us to truth and light; Honor right and conquer tvrong; As a beacon o'er dark water This the burden of our song This is for thee our prayer. Ever her truth proclaim. C .E. and James Haworth ;llrufa·mM Ol hrtb\ 9cl nn9'( 9nl '(nM ,19mM l)mlA 2»oi::in1O Ilru\21.l)M ; 9.ITTnt rri \}J)Ql'g 91\2 '(nM :9191J91 9mM 'Cnl 9W l3119ff 191\ lint rr91hliib 19n '(nM 193rl-g»oo hrtn rro2 9lclorr s\::in9 '(l)M . 9mcl\_ ff0::>n9cl 19n Ol 9mT .1n!ili 10ffort 9ffin3 rt2rns\:) -grro·m hrtn 91Jl:)'t'cl 1i'Tiq2 191\ '(l)M ls\-goo 19119 9d qmcl '(rll '(l)M ;-gff0't'\}J) 1'3U?I'f(X} Ml) lrt-gi'T 't'OffOH ;1rl-gil hrtn Mml Ol ™~rrihi»O -grro2 1»0 to rr!ili1»cl 9Ib 2is\T 1'93.J:)\}J) >\100 'T3 IQ ff0::ln30 J:) 2A . -

Licensees 2017-11-01 10:40:12 AM Licensed As of 11/2017 LSU
IMG College Licensing Licensees 2017-11-01 10:40:12 AM Licensed as of 11/2017 LSU Distribution Channel(s) Standard License Distribution Channel(s) 2 Bandits LLC (92821) Internet/TV/Catalog Campus/Local Channel Related Retail/Direct Grocery/Drug/Convenience Stores 7575 Jefferson Hwy #89 Internet/TV/Catalog Baton Rouge, LA 70806 Product Categories Specialty Mid-Tier Mr. Patrick Wilkerson 05E (Publishing) Sporting Goods/Sports Specialty/Fan Phone 225-300-8989 07H (Electronics & Content) Shops Fax 225-208-1204 www.Bbdrygoods.com Product Categories [email protected] 4imprint Inc. (49210) 04A (Automobile Products) Spec Agr Disclosed: 06/15/2017 PO Box 320 Oshkosh, WI 54903-0320 Distribution Channel(s) Absolute Foi LLC (75511) Mr. John Lord Campus/Local Channel Phone 920-236-7272 2008 Airline Dr. Suite 300-116 Related Retail/Direct Fax 920-236-7282 Bossier City, LA 71111-2947 Restricted Channel www.4imprint.com Ms. Terri Nicholas Specialty Mid-Tier [email protected] Phone 501-309-3633 Sporting Goods/Sports Specialty/Fan Fax 813-890-2974 Shops Spec Agr Disclosed: 06/14/2017 www.Absolutefoi.com Product Categories Distribution Channel(s) [email protected] 01A (Men's/Unisex T-shirts) Restricted Channel Spec Agr Disclosed: 03/31/2017 01I (Women's T-shirts) Product Categories Distribution Channel(s) 01S (Women's Fleece Tops & Bottoms) CSA (Campus Supplier Apparel) Related Retail/Direct 01W (Women's Fashion Tops) CSN (Campus Supplier Non- Apparel) Product Categories 04A (Automobile Products) 04A (Automobile Products) 04D (Gifts & Novelties - Miscellaneous) 05A (Stationery) A-Line Apparel Company LLC 05E (Publishing) (92204) 210 Brands Inc. -

Tiger Tailer Newsletter
LSU TTIIGGEERR TTAAIILLEERR NEWSLETTER NO VEM B ER 1, 2019 LSU Trademark Licensing VO LUM E 16, ISSU E 61 225‐578‐3386 Dear LSU Tiger Tailer, Nov. 29 vs. Missouri State LSU Trademark LSU Trademark Licensing hopes that Fall Fall Commencement Licensing is going well for you and your store. The Dec. 20 330 Thomas Boyd Hall LSU football team is having a fantastic Baton Rouge, LA 70803 season and the Tigers have put themselves 225-578-3386 in a good position for their first CFP berth Thank you for your continued support of LSU through the sale of Officially and potentially finishing the season in [email protected] Licensed Products. Your efforts in selling New Orleans. We will keep you up to date www.LSU.com on possible SEC Championship and bowl officially licensed LSU products have game licensing information as it becomes served to put LSU at the #7 overall Contents available. ranking in the CLC Consortium in licensing royalties generated back to the Updates 1 Upcoming Trade Shows University this fiscal year. LSU’s Top & New Licensees 2 The Sports Licensing and Tailgate Show GEAUX TIGERS! will take place at the Mandalay Bay The Northwest Company 3 Convention Center in Las Vegas from Nap Cap 4 Thursday, January 16 - Saturday, January 18, 2020. For more information on the Sports Licensing and Tailgate Show, please visit www.sportstailgateshow.com. The CAMEX trade show will take place at the Ernest Morial Convention Center in New Orleans, LA on Monday, February 10-Tuesday, February 11, 2020. To learn more about CAMEX, visit www.CAMEX.org or call 800-622-7498. -
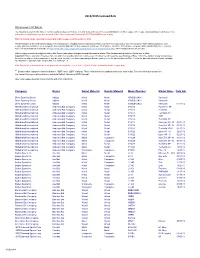
2014/2015 Licensed Bats
2014/2015 Licensed Bats 2014 Licensed 2 1/4" Bat List The following is a list of bats with a 2 1/4 inch maximum diameter that are currently licensed for use in the baseball divisions of Little League (Little League [Majors] Division and below). Bats with metal or wood barrels may also be used in the Junior League and Intermdeiate 50/70 Baseball Divisons of play. This list includes bats currently licensed with Little League as of December 8, 2014 This list includes all licensed models organized by manufacturer, including those composite barrel bats that have received a waiver from the composite-barreled bat moratorium. Little League placed a moratorium on all composite-barreled baseball bats for these divisions, which took effect on Dec. 30, 2010. A list of those composite-barreled bats that have received a waiver of that moratorium is available at http://www.littleleague.org/learn/equipment/licensedcompositebats.htm, which includes photos of each bat. Little League reserves the right to add to this list or make other changes as new information arises. This list was last updated on December 8, 2014. Important: This is only a list of licensed bats, not a list of all possible bats that could be used. Provided the bat meets the specifications of Rule 1.10 for the division of play, and provided the bat is not subject to the moratorium, it may be used. Any bat, even those appearing on this list, must meet all the specifications of Rule 1.10 for the particular division of play, including specifications regarding length, weight, diameter, markings, etc.