Eosinophilic Esophagitis Occurring After Switching to Ultra-Pasteurized Milk: Coincidence Or Unrecognized Etiologic Trigger?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Are Infants at Increased Risk of Breakthrough Reactions? Clinical Follow-Up After an Oral Induction Tolerance Protocol M
All abstracts are strictly embargoed until the date of presentation at the 2012 Annual Meeting J ALLERGY CLIN IMMUNOL Abstracts AB1 VOLUME 129, NUMBER 2 A Mutation in the Human Uncoordinated 119 Gene Impairs TCR Rapid Induction of Tolerance To Peanut By Antigen-coupled Cell 1 Signaling and is Associated with CD4 Lymphopenia. 3 Transfer M. M. Gorska1,2, R. Alam1,2; 1National Jewish Health, Denver, CO, C. Hsu, C. B. Smarr, A. J. Byrne, S. D. Miller, P. J. Bryce; Northwestern 2University of Colorado Denver, Aurora, CO. University, Chicago, IL. RATIONALE: Idiopathic CD4 Lymphopenia (ICL) is an immunodefi- RATIONALE: Food allergy is an increasing worldwide health concern. ciency syndrome of unclear etiology. Lck is a major TCR-linked kinase. Immunotherapy is an effective therapeutic approach but has a high risk of Lck activity was reported to be reduced in ICL. We hypothesized that ICL adverse reaction. New methods to efficiently and safely induce antigen- was associated with a defect of the recently described Lck activator specific tolerance could improve the clinical approach to food allergy. 1- -Uncoordinated 119 (Unc119). ethyl-3-(3’-dimethylaminopropyl)-carbodiimide (ECDI) is a non-toxic METHODS: CD4 T cells from three ICL patients were analyzed for Lck chemical used to link antigens to cells (Ag-SP) and has been used in activity (immune-complex kinase assay), the Unc119 protein level (west- clinical trials and shown to promote tolerance in Th1/Th17 autoimmune ern blotting) and sequence of the Unc119 cDNA and exons. The identified diseases. However, the tolerizing effect of Ag-SP on Th2-associated mutant cDNA was expressed in normal CD4 T cells by retroviral infection diseases is still unclear. -

Milk Allergen by the Numbers
Milk allergen component testing Bos d4 Milk Bos allergen d5 Bos by the d8 numbers Detect sensitizations to the complete milk protein to create personalized management plans for your patients. High levels of milk IgE may predict the likelihood of sensitivity, but may not be solely predictive of TC 2851 reactions to baked milk or α-lactalbumin allergy duration.1 • Susceptible to heat denaturation2 • HIGHER RISK of reaction to fresh milk1,3 • LOWER RISK of reaction Milk allergen to baked milk1,3,a • Patient likely to “outgrow” component testing milk allergy4 Measurement of specific IgE by blood test that provides objective assessment of sensitization to milk is the first step in discovering your patient’s allergy. Milk allergen component tests can help α-lactalbumin β-lactoglobulin Casein Test interpretations and next steps you determine the likelihood of reaction to baked goods, such as cookies or cheese pizza, as well as • Avoid fresh milk the likelihood of allergy persistence. + + - • Likely to tolerate baked milk products • Baked milk oral food challenge (OFC), Knowing which protein your patient is with a specialist may be appropriate sensitized to can help you develop a + - - • Likely to outgrow allergy management plan.3,5-9 - + - • Avoid all forms of milk +/- +/- + • Unlikely to become tolerant of milk over time • Avoid milk and baked milk products (yogurt, cookies, cakes), as well as products processed with milk (chocolate, sausage, potato chips) % of children with milk allergy 75 do not react to baked milk.3 Bos d4 Bos Bos d5 d8 TC 2852 TC 2853 β-lactoglobulin Casein Determine • Susceptible to heat • Resistant to heat which proteins denaturation2 denaturation3 your patient is • HIGHER RISK of reaction • HIGHER RISK of reaction to fresh milk1,3 to all forms of milk1,3,5 sensitized to. -

Modulation of Milk Allergenicity by Baking Milk in Foods: a Proteomic Investigation
nutrients Article Modulation of Milk Allergenicity by Baking Milk in Foods: A Proteomic Investigation Simona L. Bavaro 1 , Elisabetta De Angelis 1 , Simona Barni 2, Rosa Pilolli 1 , Francesca Mori 2, Elio. M. Novembre 2 and Linda Monaci 1,* 1 Institute of Sciences of Food Production, Italian National Research Council (ISPA-CNR), Via Amendola 122/O, 70126 Bari, Italy 2 Allergy Unit, Department of Pediatrics, Anna Meyer Children0s University Hospital, University of Florence, 50139 Florence, Italy * Correspondence: [email protected]; Tel.: +39-080-592-9343 Received: 31 May 2019; Accepted: 1 July 2019; Published: 6 July 2019 Abstract: Cow’s milk is considered the best wholesome supplement for children since it is highly enriched with micro and macro nutrients. Although the protein fraction is composed of more than 25 proteins, only a few of them are capable of triggering allergic reactions in sensitive consumers. The balance in protein composition plays an important role in the sensitization capacity of cow’s milk, and its modification can increase the immunological response in allergic patients. In particular, the heating treatments in the presence of a food matrix have demonstrated a decrease in the milk allergenicity and this has also proved to play a pivotal role in developing tolerance towards milk. In this paper we investigated the effect of thermal treatment like baking of cow’s milk proteins that were employed as ingredients in the preparation of muffins. A proteomic workflow was applied to the analysis of the protein bands highlighted along the SDS gel followed by western blot analyses with sera of milk allergic children in order to have deeper information on the impact of the heating on the epitopes and consequent IgE recognition. -

BSACI Guideline for the Diagnosis and Management of Cow's Milk Allergy
doi: 10.1111/cea.12302 Clinical & Experimental Allergy, 44, 642–672 BSACI GUIDELINES © 2014 John Wiley & Sons Ltd BSACI guideline for the diagnosis and management of cow’s milk allergy D. Luyt1, H. Ball1, N. Makwana2, M. R. Green1, K. Bravin1, S. M. Nasser3 and A. T. Clark3 1University Hospitals of Leicester NHS Trust, Leicester, UK, 2Sandwell and West Birmingham Hospitals NHS Trust, Birmingham, UK and 3Cambridge University Hospital NHS Foundation Trust, Cambridge, UK Summary Clinical This guideline advises on the management of patients with cow’s milk allergy. Cow’s milk & allergy presents in the first year of life with estimated population prevalence between 2% and 3%. The clinical manifestations of cow’s milk allergy are very variable in type and Experimental severity making it the most difficult food allergy to diagnose. A careful age- and disease- specific history with relevant allergy tests including detection of milk-specific IgE (by skin Allergy prick test or serum assay), diagnostic elimination diet, and oral challenge will aid in diag- nosis in most cases. Treatment is advice on cow’s milk avoidance and suitable substitute milks. Cow’s milk allergy often resolves. Reintroduction can be achieved by the graded exposure, either at home or supervised in hospital depending on severity, using a milk ladder. Where cow’s milk allergy persists, novel treatment options may include oral toler- ance induction, although most authors do not currently recommend it for routine clinical practice. Cow’s milk allergy must be distinguished from primary lactose intolerance. This Correspondence: guideline was prepared by the Standards of Care Committee (SOCC) of the British Society Dr Andy T. -

Terra Food Catalogue 250X23
We are actively expanding the geographical reach of our exports and are seeking to cooperate with reliable partners who CONTACT US value constant high quality products, a long standing professional reputation, experience and stability. We are ready to manufacture products based on customer specications and in volumes required. The wealthy natural resources of Ukraine and many years of fruitful cooperation with leading ingredients suppliers provide us with a solid production base. Besides, at our clients’ disposal are always the best traditions of Ukrainian butter makers, their vast experience and passion for healthy and tasty food. Our customers around the world are companies producing high-quality products. Please contact our Export Sales Department regarding partnership and our products distribution: Tel.: + 380 44 594 70 33 E-mail: [email protected] terrafood-export.com 2015 TERRA FOOD GROUP is a leading The Group is Ukrainian market leader in butter, spread Ukrainian dairy manufacturing company. and cheese and one of the three largest producers in Russia, as well as the largest spread exporter in the CIS, As one of the largest Ukrainian exporters of spreads the Middle East and North Africa. We are interested in and cheeses, we export our products to 27 countries expanding our own brands (FERMA, Gold Valley, around the world. TERRA FOOD has been selling Premialle and others) into new markets and are eager high-quality products to the Middle East, North Africa to cooperate with distributors on favorable terms. and Asia, Azerbaijan, Georgia, Armenia, Kazakhstan and Turkmenistan, as well as to Moldova, the Balkans, Russia for over 10 years. -

Customs Union Technical Regulation on Milk and Dairy Products Russian
THIS REPORT CONTAINS ASSESSMENTS OF COMMODITY AND TRADE ISSUES MADE BY USDA STAFF AND NOT NECESSARILY STATEMENTS OF OFFICIAL U.S. GOVERNMENT POLICY Voluntary - Public Date: 11/18/2013 GAIN Report Number: RS1382 Russian Federation Post: Moscow Customs Union Technical Regulation on Milk and Dairy Products Report Categories: Trade Policy Monitoring Sanitary/Phytosanitary/Food Safety FAIRS Subject Report Dairy and Products Approved By: Christopher Riker Prepared By: Staff Report Highlights: The Technical Regulation of the Russia-Kazakhstan-Belarus Customs Union (CU) “On Safety of Milk and Dairy Products” (TR TS 033/2013) is a key CU regulation covering standards and requirements for milk and dairy products. This Technical Regulation was adopted by Decision of the Council of the Eurasian Economic Commission No. 67 of October 9, 2013, and will come into effect as of May 1, 2014. General Information The Technical Regulation of the Russia-Kazakhstan-Belarus Customs Union (CU) “On Safety of Milk and Dairy Products” (TR TS 033/2013) is a key CU regulation covering standards and requirements for milk and dairy products. This Technical Regulation was adopted by Decision of the Council of the Eurasian Economic Commission No. 67 of October 9, 2013, and will come into effect as of May 1, 2014. Below is an unofficial translation of the following: - Decision of the Council of the Eurasian Economic Commission No.67 of October 9,2013; - CU Technical Regulation “On Safety of Milk and Dairy Products” (TR TS 033/2013) with 16 annexes; BEGIN UNOFFICIAL TRANSLATION: DECISION October 9, 2013 No. 67 Kazan On the Technical Regulation of the Customs Union “On Safety of Milk and Dairy Products” In accordance with Article 3 of the Agreement on the Eurasian Economic Commission of November 18, 2011, the Council of the Eurasian Economic Commission has decided : 1. -
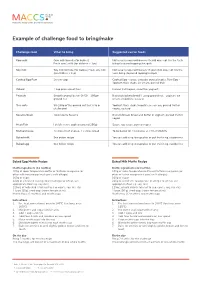
Example of Challenge Food to Bring/Make
Example of challenge food to bring/make Challenge food What to bring Suggested carrier foods Cow milk Cow milk formula (for babies) Not usually required however if child does not like the taste Fresh cow’s milk (for children > 1yo) bring flavoured toppings/nesquik Soy milk Soy milk formula (for babies) Fresh soy milk Not usually required however if your child does not like the (for children > 1yo) taste bring flavoured topping/nesquik Cooked Egg Raw 2x raw eggs Cooked Egg - sauce, avocado, pureed vegies, Raw Egg - Yoghurt, thick shake, ice cream, pureed fruit Wheat 1 cup plain wheat flour Pureed fruit/vegies, smoothie, yoghurt, Peanuts Smooth peanut butter Or 50 – 100gm Biscuits/crackers/bread If using ground nut – yoghurt, ice ground nut cream, smoothies, custard Tree nuts 50-100g of the ground nut that is to be Yoghurt, thick shake/smoothie, ice-cream, pureed fruit or challenged vegies, custard Sesame Seed Tahini paste Sesame Biscuits/bread, Bread and butter or yoghurt, pureed fruit or vegies Meat/Fish I child’s serve: cooked (around 250g) Sauce, soy sauce, pureed vegies Melted cheese 2x slices Kraft cheese, 2 x slices bread To be baked for 13 minutes at 220 at MACCS Baked milk See below recipe You can add icing to cupcakes or put fruit in eg, raspberries Baked egg See below recipe You can add icing to cupcakes or put fruit in eg, raspberries Baked Egg Muffin Recipe Baked Milk Muffin Recipe Muffin ingredients (12 muffins) Muffin ingredients (12 muffins) 100g of room temperature butter or Nuttelex margarine (or 100g of room temperature butter or Nuttelex margarine (or other milk free margarine if cow’s milk allergic) other milk free margarine if cow’s milk allergic) 160g of sugar 160g of sugar 220g of sifted self-raising flour (if allergic to wheat, use 220g of sifted self-raising flour (if allergic to wheat, use appropriate flour e.g. -

Cow's Milk Processing—Friend Or Foe in Food Allergy?
foods Review Cow’s Milk Processing—Friend or Foe in Food Allergy? Sabine Geiselhart †, Aleksandra Podzhilkova † and Karin Hoffmann-Sommergruber * Department of Pathophysiology and Allergy Research, Medical University of Vienna, 1090 Vienna, Austria; [email protected] (S.G.); [email protected] (A.P.) * Correspondence: [email protected] † These authors contributed equally to this work. Abstract: Cow’s milk (CM) is an integral part of our daily diet starting in infancy and continuing throughout our lifetime. Its composition is rich in proteins with a high nutritional value, bioactive components, milk minerals including calcium, and a range of immunoactive substances. However, cow’s milk can also induce a range of immune-mediated diseases including non-IgE-mediated food allergies and IgE-mediated food allergies. Cow’s milk allergens have been identified and char- acterized and the most relevant ones can be assigned to both, the whey and casein fraction. For preservation a range of processing methods are applied to make cow’s milk and dairy products safe for consumers. However, these methods affect milk components and thus alter the overall immuno- genic activity of cow’s milk. This review summarizes the current knowledge on cow’s milk allergens and immunoactive substances and the impact of the different processes up- or downregulating the immunogenicity of the respective proteins. It highlights the gaps of knowledge of the related disease mechanisms and the still unidentified beneficial immunomodulating compounds of cow’s milk. Keywords: cow’s milk allergens; whey; casein; food processing; IgE-mediated food allergy Citation: Geiselhart, S.; Podzhilkova, A.; Hoffmann-Sommergruber, K. -

Food Challenge Booklet
Food Challenge Booklet Patient Sticker: Algorithm for challenge Tick Assess child is well (not pyrexial, no wheeze, no recent reaction) Steroid use?/Salbutamol use? Not taken cetirizine for at least 5 days /Chlorpheniramine 2 days Consent signed If unwell, taking antihistamines or Proceed with food challenge/ +/- current exacerbation of asthma- rebook Skin prick test (repeat SPT if nut challenge for another day and last one more than 6 months ago) Allergic reaction No allergic reaction -Stop procedure -Observe for 2 hours after final dose -Treat as required -Advice on incorporation of allergen into diet -Observe -Give advice leaflet -Avoid allergen- same as pre challenge -Review requirements for follow up -Give treatment plan -Complete Discharge Summary -Complete discharge Summary Drugs to be prescribed on drug chart from BNFC 2016 < 30 kg Adrenaline Autoinjector 150 mcg Adrenaline >30 kg Adrenaline Autoinjector 300mcg Anti-histamine Cetirizine If cetirizine fails, Chlorphenramine < 2yrs 0.25mg/kg 1mg 2-5 yrs 2.5mg 1mg 6-12yrs 5mg 2mg >12yrs 10mg 4mg Salbutamol <5yr 2.5mg nebulized or 10puffs >5 yr 5mg nebulised or 10 puffs Drug/ Food Proforma Patient sticker: Date Patient Name Hospital Number DOB Food/drug being challenged SPT/IgE results to relevant food (positive and Food + Positive -Negative negative control results) Previous reaction Other allergies Asthma YES NO If yes any symptoms in last week? Last salbutamol use? Taking preventer? Allergic Rhinitis YES NO Eczema YES NO If yes where affected Medications Epipens? (in date) Drug -

Download the Catalog
MILK Kind of Quantity Box Article Name % of packa- Weight, in box, weight, Storage Shelf life code fat ging kg pcs. kg conditions PASTEURIZED 11102 MILK 1.5 Sachets 900 12 10,80 0 ⁰С - + 6 ⁰С 8 PASTEURIZED 11117 MILK 2,7 Sachets 900 12 10,80 +2 ⁰С - + 6 ⁰С 8 PASTEURIZED 11123 MILK 3.2 Sachets 900 12 10,80 0 ⁰С - + 6 ⁰С 8 PASTEURIZED 11115 MILK 2,7 Sachets 450 20 9,00 +2 ⁰С - + 6 ⁰С 8 PASTEURIZED 11121 MILK 3,2 Sachets 450 20 9,00 0 ⁰С - + 6 ⁰С 8 11202 BAKED MILK 2,5 Sachets 450 20 9,00 0 ⁰С - + 6 ⁰С 8 PASTEURIZED 11119 MILK 2.7 Ecolean 900 6 5,40 +2 ⁰С - + 6 ⁰С 10 11204 BAKED MILK 2,5 Ecolean 900 6 5,40 0 ⁰С - + 6 ⁰С 10 FERMENTED MILK PRODUCTS Quantity Article Name % of fat Kind of Weight, in box, Box weight, Storage Shelf life code packaging kg pcs. kg conditions 12136 KEFIR 0 Sachets 900 12 10,80 +2⁰С - + 6⁰С 14 12112 KEFIR 1 Sachets 900 12 10,80 +2⁰С - + 6⁰С 14 12121 KEFIR 2,5 Sachets 900 12 10,80 +2⁰С - + 6⁰С 14 12104 KEFIR 0 Sachets 450 20 9,00 +2⁰С - + 6⁰С 14 12110 KEFIR 1 Sachets 450 20 9,00 +2⁰С - + 6⁰С 14 12119 KEFIR 2,5 Sachets 450 20 9,00 +2⁰С - + 6⁰С 14 12125 KEFIR 3,2 Sachets 450 20 9,00 +2⁰С - + 6⁰С 14 12103 KEFIR 0 Ecolean 900 6 5,40 +2⁰С - + 6⁰С 14 12137 KEFIR 1 Ecolean 900 6 5,40 +2⁰С - + 6⁰С 14 12117 KEFIR 2,5 Ecolean 900 6 5,40 +2⁰С - + 6⁰С 14 KEFIR 12134 “NASH” 2,5 Sachets 450 20 9,00 0⁰С - + 6⁰С 3 KEFIR 12130 “ZHYVYI” 2,5 Sachets 450 20 9,00 0⁰С - + 6⁰С 3 FERMENTED 12303 BAKED MILK 2,5 Sachets 450 20 9,00 0⁰С - + 6⁰С 14 FERMENTED 12305 BAKED MILK 2,5 Ecolean 450 10 4,50 0⁰С - + 6⁰С 14 12402 WHEY 0 Sachets 900 12 10,80 -

Component-Resolved Diagnosis in Food Allergies
medicina Review Component-Resolved Diagnosis in Food Allergies Elisabetta Calamelli 1,*, Lucia Liotti 2, Isadora Beghetti 3 , Valentina Piccinno 1, Laura Serra 1 and Paolo Bottau 1 1 Pediatric and Neonatology Unit, Imola Hospital, 40026 Imola, Italy 2 Pediatric Unit, Civic Hospital, 60019 Senigallia, Italy 3 Pediatric Unit, Department of Medical and Surgical Sciences (DIMEC), S.Orsola-Malpighi Hospital, University of Bologna, 40138 Bologna, Italy * Correspondence: [email protected]; Tel.: +39-0542-662807 Received: 22 May 2019; Accepted: 15 August 2019; Published: 18 August 2019 Abstract: Component-resolved diagnostics (CRD) in food allergies is an approach utilized to characterize the molecular components of each allergen involved in a specific IgE (sIgE)-mediated response. In the clinical practice, CRD can improve diagnostic accuracy and assist the physician in many aspects of the allergy work-up. CRD allows for discriminatory co-sensitization versus cross-sensitization phenomena and can be useful to stratify the clinical risk associated with a specific sensitization pattern, in addition to the oral food challenge (OFC). Despite this, there are still some unmet needs, such as the risk of over-prescribing unnecessary elimination diets and adrenaline auto-injectors. Moreover, up until now, none of the identified sIgE cutoff have shown a specificity and sensitivity profile as accurate as the OFC, which is the gold standard in diagnosing food allergies. In light of this, the aim of this review is to summarize the most relevant concepts in the field of CRD in food allergy and to provide a practical approach useful in clinical practice. Keywords: alfa-gal; casein; gliadin; lipid transfer protein; ovomucoid; parvalbumin; tropomyosin 1. -

Home Baked Milk Introduction a Guide for Parents and Children Seen in Our Allergy Service
Home baked milk introduction A guide for parents and children seen in our allergy service This information sheet will guide you through the process of home baked milk introduction. This process involves gradual introduction of baked milk, starting from a small dose and increasing the amount over the period of approximately three months. What does extensively heated cow’s milk mean? [baked milk] This means baking cow’s milk at 180C for 20 minutes in order to change the structure of the cow’s milk protein. Why we are recommending home baked cow’s milk introduction We have performed allergy tests which indicate that your child has a good chance of tolerating extensively heated cow’s milk. This suggests that you could gradually introduce baked milk into your child’s diet under your supervision after which your child should be able to consume foods containing baked cow’s milk without having an allergic reaction. When to perform the home baked cow’s milk introduction Make sure your child is well with no illness (including mild coughs and colds). Make sure that their eczema, asthma or hay-fever has not flared up. Your child can continue to take their regular antihistamines (for example, Piriton®, Cetirizine, Loratidine) throughout home desensitisation. Where to perform home baked cow’s milk introduction Make sure that the first time you give the food and each time you increase the amount of food you give to your child, you do this in your own home (not at nursery or school). You will need to allow sufficient time to observe your child for at least two hours after the first dose and after each of the first increased doses of baked cow’s milk.