Variable Movements After Catch-And-Release Tournament Angling and Isotopic Niches
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
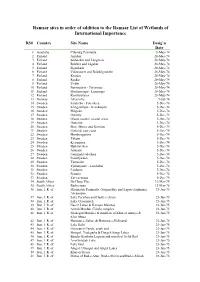
Ramsar Sites in Order of Addition to the Ramsar List of Wetlands of International Importance
Ramsar sites in order of addition to the Ramsar List of Wetlands of International Importance RS# Country Site Name Desig’n Date 1 Australia Cobourg Peninsula 8-May-74 2 Finland Aspskär 28-May-74 3 Finland Söderskär and Långören 28-May-74 4 Finland Björkör and Lågskär 28-May-74 5 Finland Signilskär 28-May-74 6 Finland Valassaaret and Björkögrunden 28-May-74 7 Finland Krunnit 28-May-74 8 Finland Ruskis 28-May-74 9 Finland Viikki 28-May-74 10 Finland Suomujärvi - Patvinsuo 28-May-74 11 Finland Martimoaapa - Lumiaapa 28-May-74 12 Finland Koitilaiskaira 28-May-74 13 Norway Åkersvika 9-Jul-74 14 Sweden Falsterbo - Foteviken 5-Dec-74 15 Sweden Klingavälsån - Krankesjön 5-Dec-74 16 Sweden Helgeån 5-Dec-74 17 Sweden Ottenby 5-Dec-74 18 Sweden Öland, eastern coastal areas 5-Dec-74 19 Sweden Getterön 5-Dec-74 20 Sweden Store Mosse and Kävsjön 5-Dec-74 21 Sweden Gotland, east coast 5-Dec-74 22 Sweden Hornborgasjön 5-Dec-74 23 Sweden Tåkern 5-Dec-74 24 Sweden Kvismaren 5-Dec-74 25 Sweden Hjälstaviken 5-Dec-74 26 Sweden Ånnsjön 5-Dec-74 27 Sweden Gammelstadsviken 5-Dec-74 28 Sweden Persöfjärden 5-Dec-74 29 Sweden Tärnasjön 5-Dec-74 30 Sweden Tjålmejaure - Laisdalen 5-Dec-74 31 Sweden Laidaure 5-Dec-74 32 Sweden Sjaunja 5-Dec-74 33 Sweden Tavvavuoma 5-Dec-74 34 South Africa De Hoop Vlei 12-Mar-75 35 South Africa Barberspan 12-Mar-75 36 Iran, I. R. -

Saskatchewan Birding Trail Experience (Pdf)
askatchewan has a wealth of birdwatching opportunities ranging from the fall migration of waterfowl to the spring rush of songbirds and shorebirds. It is our hope that this Birding Trail Guide will help you find and enjoy the many birding Slocations in our province. Some of our Birding Trail sites offer you a chance to see endangered species such as Piping Plovers, Sage Grouse, Burrowing Owls, and even the Whooping Crane as it stops over in Saskatchewan during its spring and fall migrations. Saskatchewan is comprised of four distinct eco-zones, from rolling prairie to dense forest. Micro-environments are as varied as the bird-life, ranging from active sand dunes and badlands to marshes and swamps. Over 350 bird species can be found in the province. Southwestern Saskatchewan represents the core of the range of grassland birds like Baird's Sparrow and Sprague's Pipit. The mixed wood boreal forest in northern Saskatchewan supports some of the highest bird species diversity in North America, including Connecticut Warbler and Boreal Chickadee. More than 15 species of shorebirds nest in the province while others stop over briefly en-route to their breeding grounds in Arctic Canada. Chaplin Lake and the Quill Lakes are the two anchor bird watching sites in our province. These sites are conveniently located on Saskatchewan's two major highways, the Trans-Canada #1 and Yellowhead #16. Both are excellent birding areas! Oh! ....... don't forget, birdwatching in Saskatchewan is a year round activity. While migration provides a tremendous opportunity to see vast numbers of birds, winter birding offers you an incomparable opportunity to view many species of owls and woodpeckers and other Arctic residents such as Gyrfalcons, Snowy Owls and massive flocks of Snow Buntings. -

Healthy Beaches Report
Saskatchewan Recreational Water Sampling Results to July 8, 2019 Water is Caution. Water Water is not Data not yet suitable for quality issues suitable for available/Sampling swimming observed swimming complete for season Legend: Recreational water is considered to be microbiologically safe for swimming when single sample result contains less than 400 E.coli organisms in 100 milliliters (mLs) of water, when the average (geometric mean) of five samples is under 200 E.coli/100 mLs, and/or when significant risk of illness is absent. Caution. A potential blue-green algal bloom was observed in the immediate area. Swimming is not recommended; contact with beach and access to facilities is not restricted. Resampling of the recreational water is required. Swimming Advisory issued. A single sample result containing ≥400 E.coli/100 mLs, an average (geometric mean) of five samples is >200 E.coli/100 mLs, an exceedance of the guideline value for cyanobacteria or their toxins >20 µg/L and/or a cyanobacteria bloom has been reported. Note: Sampling is typically conducted from June – August. Not all public swimming areas in Saskatchewan are monitored every year. Historical data and an annual environmental health assessment may indicate that only occasional sampling is necessary. If the quality of the area is deteriorating, then monitoring of the area will occur. This approach allows health officials to concentrate their resources on beaches of questionable quality. Every recreational area is sampled at least once every five years. Factors affecting the microbiological quality of a water body at any given time include type and periodicity of contamination events, time of day, recent weather conditions, number of users of the water body and, physical characteristics of the area. -

Blue Jay, Vol.37, Issue 2
THE PIPING PLOVER IN SASKATCHEWAN: A STATUS REPORT WAYNE E. RENAUD, LGL Ltd. — environmental research associates, 4' Eglinton Ave. West, Toronto, Ontario M4R 1A1, GUY J. WAPPLE, Box 1153 Biggar, Saskatchewan SOK 0M0, and DURAND W. EDGETT, 628 Church St. Apt. 4, Toronto, Ontario M4Y 2G3. The Piping Plover is the only small Society’s “Blue List” of threatene* plover that breeds in a large area of species since its inception in 1972.3- southern Canada and the northern This paper briefly summarizes th< United States. Its breeding range is species’ status in Canada, anc divided, probably by habitat brings together all existing infor availability, into three areas: the mation on its occurrence in Saskaf; Atlantic coast from Virginia to chewan. Newfoundland, the Great Lakes, and the western plains from central Status in Canada Alberta and Manitoba to Nebraska.2 Declines since the 1930’s havo The Piping Plover nests in a variety been most severe along the Grea of habitats including ocean beaches, Lakes. In the late 1800’s and earl sand dunes, river bars, and the 1900’s, Piping Plovers nested alonr shores of lakes, alkaline sloughs and the Canadian shorelines fron reservoirs. Kingston to the Bruce Peninsula.2 During the past 100 years, the The largest breeding population wa Piping Plover has experienced apparently at Long Point, a 29-km population declines over a large por¬ long peninsula on the north shore a tion of its range. In the late 1800’s, Lake Erie. Snyder estimated that a spring hunting in New England least 100 pairs nested there in 193CI greatly reduced the numbers of and Sheppard counted up to 5( Piping Plovers breeding along the adults in one day during Jul; Atlantic coast.11 This practice had 1935.40 39 Numbers at Long Poin! largely ceased by the early 1900’s, declined during the 1960’s am and their numbers slowly increased. -

Lake Diefenbaker: the Prairie Jewel
Journal of Great Lakes Research 41 Supplement 2 (2015) 1–7 Contents lists available at ScienceDirect Journal of Great Lakes Research journal homepage: www.elsevier.com/locate/jglr Lake Diefenbaker: The prairie jewel Introduction with intakes that are 34 m below FSL. The proportion of inflowing water that flows out of Gardiner Dam varies from ~70% in low flow years (e.g., 2001 average flow was 84 m3 s−1)toover97%inhigh fl 3 −1 Fighting drought with a dam: Lake Diefenbaker, the reservoir of ow years (e.g., 2005; 374 m s ; Hudson and Vandergucht, 2015) fl life-giving water.CBC's Norman DePoe. with a median proportional out ow of ~94%. Additional water losses in- clude net evaporation, which can represent over 10% in dry years, irriga- On the broad expanse of flat land of the northern Great Plains of tion, and direct use from the reservoir. From the Qu'Appelle Dam, Lake Canada lies a large, narrow, river-valley reservoir, Lake Diefenbaker. Diefenbaker water is transferred out of the natural catchment and re- The reservoir was developed for multiple benefits including the irriga- leased down the Qu'Appelle River to supply southern regions of the tion of ~2000 km2 of land in Central Saskatchewan, the generation of province. With a mean depth of 22 m, the water residence time, though hydroelectricity, a source of drinking water, flood control, water for in- variable, is typically just over a year (Donald et al., 2015; Hudson and dustry and livestock, and recreation. This semi-arid region has an aver- Vandergucht, 2015). -

2017 Anglers Guide.Cdr
Saskatchewan Anglers’ Guide 2017 saskatchewan.ca/fishing Free Fishing Weekends July 8 and 9, 2017 February 17, 18 and 19, 2018 Minister’s Message I would like to welcome you to a new season of sport fishing in Saskatchewan. Saskatchewan's fishery is a priceless legacy, and it is the ministry's goal to maintain it in a healthy, sustainable state to provide diverse benefits for the province. As part of this commitment, a portion of all angling licence fees are dedicated to enhancing fishing opportunities through the Fish and Wildlife Development Fund (FWDF). One of the activities the FWDF supports is the operation of Scott Moe the Saskatchewan Fish Culture Station, which plays a Minister of Environment key role in managing a number of Saskatchewan's sport fisheries. To meet the province's current and future stocking needs, a review of the station's aging infrastructure was recently completed, with a multi-year plan for modernization and refurbishment to begin in 2017. In response to the ongoing threat of aquatic invasive species, the ministry has increased its prevention efforts on several fronts, including increasing public awareness, conducting watercraft inspections and monitoring high- risk waters. I ask everyone to continue their vigilance against the threat of aquatic invasive species by ensuring that your watercraft and related equipment are cleaned, drained and dried prior to moving from one body of water to another. Responsible fishing today ensures fishing opportunities for tomorrow. I encourage all anglers to do their part by becoming familiar with this guide and the rules and regulations that pertain to your planned fishing activity. -

Water Quality in the South SK River Basin
Water Quality in the South SK River Basin I AN INTRODUCTION TO THE SOUTH SASKATCHEWAN RIVER BASIN I.1 The Saskatchewan River Basin The South Saskatchewan River joins the North Saskatchewan River to form one of the largest river systems in western Canada, the Saskatchewan River System, which flows from the headwater regions along the Rocky Mountains of south-west Alberta and across the prairie provinces of Canada (Alberta, Saskatchewan, and Manitoba). The Prairie physiographic region is characterized by rich soils, thick glacial drift and extensive aquifer systems, and a consistent topography of broad rolling hills and low gradients which create isolated surface wetlands. In contrast, the headwater region of the Saskatchewan River (the Western Cordillera physiographic region) is dominated by thin mineral soils and steep topography, with highly connected surface drainage systems and intermittent groundwater contributions to surface water systems. As a result, the Saskatchewan River transforms gradually in its course across the provinces: from its oxygen-rich, fast flowing and highly turbid tributaries in Alberta to a meandering, nutrient-rich and biologically diverse prairie river in Saskatchewan. There are approximately 3 million people who live and work in the Saskatchewan River Basin and countless industries which operate in the basin as well, including pulp and paper mills, forestry, oil and gas extraction, mining (coal, potash, gravel, etc.), and agriculture. As the fourth longest river system in North America, the South Saskatchewan River Basin covers an incredibly large area, draining a surface of approximately 405 860 km² (Partners FOR the Saskatchewan River Basin, 2009). Most of the water that flows in the Saskatchewan River originates in the Rocky Mountains of the Western Cordillera, although some recharge occurs in the prairie regions of Alberta and Saskatchewan through year-round groundwater contributions, spring snow melt in March or April, and summer rainfall in May and early July (J.W. -

State of Lake Diefenbaker Report
State of Lake Diefenbaker Prepared for: Consultation Meeting on May 30, 2012. Document was edited and revised on October 19, 2012 Executive Summary The purpose of this report is to provide the stakeholders with some context of the South Saskatchewan River project, the management issues associated with the operation of the Gardiner and Qu’Appelle River Dams, and the health of Lake Diefenbaker and the Lake Diefenbaker Watershed. This report summarizes the management activities associated with the operation of the South Saskatchewan River Project and the potential outcomes related to these management activities. Information within this report provides a basis for evaluating the management objectives and setting priorities for the operation of the Project. Table 1 outlines the various reservoir management activities and the resulting consequences of these activities. The South Saskatchewan River Project, of which Lake Diefenbaker and the Gardiner and Qu’Appelle River Dams are the primary components, is a critical water resource for the province of Saskatchewan. The South Saskatchewan River Project is currently owned and managed by the Water Security Agency of Saskatchewan for multiple services, including irrigation, municipal and industrial water supply, hydroelectric power generation, recreation, aquatic and wildlife habitat, and downstream flood control. The services provided by the Project are fundamental to the province’s economic, social and environmental well being. Lake Diefenbaker construction started after an agreement between the province of Saskatchewan and the Government of Canada, which was signed in 1958. The initial purpose of the project was to form a reservoir that could provide source water to irrigate approximately 200,000 hectares of farmland in central Saskatchewan and the Qu’Appelle Valley. -

Blue Jay, Vol.45, Issue 4
WILDLIFE '87: GAINING MOMENTUM P.S. TAYLOR, 1714 Prince of Wales Avenue, Saskatoon, Saskatchewan. S7K 3E5 Environment Minister Tom McMillan Wildlife '87 aims were to recognize the designated 1987 “a year of wildlife con¬ importance of wildlife conservation in servation in Canada." It was to mark 100 Canada, to increase public awareness of years since the establishment, by Sir John our wildlife heritage, to establish new con¬ A. MacDonald on 8 June 1887, of the first servation projects, to protect and set aside bird sanctuary in Canada at Last Mountain important wild areas and to involve Lake, Saskatchewan. Although the idea of people young and old in conservation dedicating the year to wildlife was en¬ projects. dorsed by provincial and territorial wildlife ministers, it was the non¬ Wildlife '87 Newsletters recorded the government conservation groups which events. Two new naturalist clubs were took the initiative and played a lead role formed in New Brunswick in January. in making "Wildlife '87" a success. The Symposia on Bear-People Conflicts, Nor¬ Canadian Nature Federation coordinated thern Forest Owls, the Fraser River and communicated with participating Estuary, Canada's Wetlands Ecology and groups across Canada. In February 1986, Conservation, and Workshops on Bird a national committee chaired by Joy Finlay Banding, and Urban Natural Areas were of Edmonton began to bring attention to held. Bald Eagle and Peregrine Falcon the many conservation efforts planned for release projects were undertaken. The Boy 1987. Scouts were awarded the World Conser- Award presentation to Wildlife '87 Chairperson Joy Finlay by Prince Philip Bob Lane 192 Blue Jay vation Badge. -

Assessment of the Instream Flow Needs for Fish and Fish Habitat in the Saskatchewan River Downstream of the E.B
Canadian Science Advisory Secretariat Central and Arctic Region Science Advisory Report 2018/048 ASSESSMENT OF THE INSTREAM FLOW NEEDS FOR FISH AND FISH HABITAT IN THE SASKATCHEWAN RIVER DOWNSTREAM OF THE E.B. CAMPBELL HYDROELECTRIC STATION Figure 1. Map of study site locations in the Figure 2. Small bodied fish stranded at ~6:50 h, Saskatchewan River, Saskatchewan (from Enders July 21, 2005 in a Site 2 side channel (photo et al. 2017) credit: D. Watkinson) Context: The E.B. Campbell Hydroelectric Station, owned and operated by SaskPower, is a hydropeaking facility on the Saskatchewan River (Figure 1). Fisheries and Oceans Canada (DFO) Fisheries Protection Program (FPP) is seeking science advice on Instream Flow Needs (IFN) to help inform a new Fisheries Act authorization, including measures to avoid, mitigate, and as necessary, offset serious harm to fish and fish habitat as a result of the ongoing operations of the existing facility. The present Fisheries Act authorization includes a minimum flow release of 75 m3·s-1 and was followed by a research study (conducted April 2005 to March 2007) to evaluate impacts on fish habitat. The results from this study, as well as additional reports, data, and publications were summarized in an unpublished 2008 DFO report. In March 2012, DFO provided national guidance on IFN (i.e., +/- 10% of instantaneous flow; 30% of mean annual discharge), however, cautioned that when data are available, a more detailed technical examination is required on the effectiveness of the recommended thresholds, in particular for cases of hydropeaking (DFO 2013). FPP has requested DFO Science to update the unpublished 2008 DFO report with a description of E.B. -

Saskatoon to Regina Via Lake Diefenbaker Saskatoon to Regina Via Lake Diefenbaker
Saskatoon to Regina via Lake Diefenbaker Saskatoon to Regina via Lake Diefenbaker SAILS, SHORES, AND SHIFTING SANDS Saskatoon to Regina via Lake Diefenbaker While Hwy #11 is the main thoroughfare between Saskatoon and Regina, an alternate route just to the west takes you through significantly different countryside including sand hills, wildlife refuges, three provincial parks, one of the world’s largest earth-filled dams, impres- sive sand dunes, and southern Saskatchewan’s largest lake. Most of this route is paved, with the exception of a few access roads. From Saskatoon, begin by taking Hwy #219 south, known as the Chief Whitecap Trail. About 13 km south of the city, you come to Beaver Creek Conservation Area where Beaver Creek meets the South Saskatchewan River. Displays in the interpre- tive centre introduce you to the flora and fauna you might see while walking the 8 km of trails that wind through natural prairie, forested valley slopes, along the mean- dering creek, and beside the sandy riverbank. South of Beaver Creek, the terrain is marked by hummocky sand hills with low shrubs and wooded coulees, and is mostly used for pasture. About 26 km south of Saskatoon, the highway passes through the Whitecap Dakota First Nation, famous for the Dakota Dunes Golf Links, rated among Canada’s top golf courses. Watch for the turn-off to the west to Round Prairie Cemetery, about 19 km south of Whitecap. The Round Prairie area was settled in the 1850s by makes a sharp turn to the west, ending at the junction with Hwy #44 Métis, and was one of the larger Métis settlements (N 51.28501 W 106.82378), just east of Gardiner Dam. -

B + 1 ,EE;Trr;Ment Environnement Fam*~1Coz’Rj3 --Hiericm Wérhdf Canada Canadian Wildlife Service Canadien Service De La Faune Printed September 1996 Ottawa, Ontario
STRATEGIC OVERVIEW OF THE CANADIAN RAMSAR PROGRAM 7 1 0 B + 1 ,EE;trr;ment Environnement fAm*~1COz’rj3 --hiericm WérhdF Canada Canadian Wildlife Service canadien Service de la faune Printed September 1996 Ottawa, Ontario This document, Strategic Overview of the Canadian Ramsar Program, has been produced as a discussion paper for Ramsar site managers and decision makers involved in the implementation of the Ramsar Convention within Canadian jurisdictions. The paper provides a general overview of the development, current status and opportunities for the future direction of the Ramsar program in Canada . Comments and suggestions on the content of this paper are welcome at the address below. Copies of this paper are available from: ® Habitat Conservation Division Canadian Wildlife Service Environment Canada Ottawa, Ontario K1 A OH3 it Phone: (819) 953-0485 Fax: (819) 994-4445 Également disponible en français. NpA ENTq4 0~ec 50% recycled paper including 10% poet- wneumer fibre. %ue 0e 50 p" 100 CIO pepier recyclé dwrt 10 p. 100 de fibres post . consOmmetiorn STRATEGIC OVERVIEW OF THE CANADIAN RAMSAR PROGRAM Prepared by: Clayton D.A. Rubec and Manjit Kerr-Upal September 1996 Habitat Conservation Division Canadian Wildlife Service Environment Canada TABLE OF CONTENTS The Ramsar Convention . .1 Ramsar in North America . : . 2 Ramsar in Canada . .2 Canada's Ramsar Database. .. .3 Distribution of Canada's Ramsar Sites . .. ... .. .. .. .. .. .. .. .3 Jurisdictional Distribution . .. .. 4 Ecozonal and Ecoregional Distributicn . .- . .- 4 Wetland Regions Distribution . 7 Wetland Classification Analysis . 8 Selection Criteria . 9 Management of Canadian Ramsar Sites . 9 Responsible Authorities for Ramsar in Canada . 13 Considerations for a National Ramsar Committee for Canada .