H3O+ Tof-CIMS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Proton Transfer Reaction in Water: Hydronium Ion Formation
Advanced Journal of Chemistry-Section A, 2020, 3(3), 255–258 Available online at : www.ajchem-a.com ISSN Online: 2645-5676 DOI: 10.33945/SAMI/AJCA.2020.3.2 Short Communication Proton Transfer Reaction in Water: Hydronium Ion Formation In Sang Leea, Sitansu Sekhar Nandab,* a Research Division, Divine Lab Limited, Seoul, South Korea b Department of Chemistry, Myongji University, Yongin, South Korea A R T I C L E I N F O A B S T R A C T Received: 28 June 2019 The current ongoing scientific debate deals with accumulation of hydronium Revised: 07 August 2019 ions (H3O+) on water surface. Elevated interfacial concentration measured by Accepted: 31 August 2019 using Raman spectroscopy. A strong surface affinity of H3O+ indicated by Raman Available online: 03 September 2019 spectroscopy under similar conditions. Ion adsorption phenomena, H3O+ formation and its structural activity emphasized in our study. Asymmetric water ion adsorption clearly observed in our research. K E Y W O R D S Raman spectroscopy Hydronium Water Infrared spectroscopy G R A P H I C A L A B S T R A C T * Corresponding author's E-mail address: [email protected] Proton Transfer Reaction in Water: Hydronium… 256 Introduction employed for recording Raman spectra with 2– 10 s acquisition time. A scanning area of 30 μm × In nature, interfacial charge formation is 30 μm was applied to avoid laser damage to known as a ubiquitous phenomenon. water. Fundamental mechanism of interfacial charge formation related with its identification. Results and discussions Researchers have been attracted for charge formation development of appropriate models. -

A Study of Hydrogen Exchange in Benzene And
A STUDY OF HYDROGEN EXCHANGE IN BENZENE AND SEVERAL ALKYLBENZENES By REX LYNN ELMORE B~chelor of Arts Austin College Sherman, Texas 1960 Submitted to the faculty of the Graduate School of the Oklahpma State University in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY August, 1965 J OKLAHOMA Jjf STATE UNIVERSITY .;.;, i' LIBRARY f DEC 6 1965 j' A STUDY OF HYDROGEN EXCHANGE IN BENZE~ AND ,,:.-· ;" . ·..... : SEVERAL ALKYLBENZENES f ~~~~~~~"'~"''""i;·~l'J!'~~ Thesis Approved: 593417 ii .ACKNOWLEDGMENTS Grateful acknowledgment is made to-Dr. E. M. Hodnett, research adviser, for his patience, guidance, and assistance throughout this work; to Dr. O. C. Dermer, head of the Chemistry Department, for his reading of the manuscript prior to its being typed; to Mr. C.R. Williams, formerly in the Chemistry Department, for his assistance in the early phases of the computer programming; to Mr. Preston Gant, Central Research Division of the Continental Oil Company, for helpful suggestions on the tritium assays; and to Mr. Prem S. Juneja for his helpful suggestions concerning experimental details which arose from time to time. Acknowledgment is made of financial assistance in the form of a graduate teaching assistantship in the Chemis.try Department. Also, a research as.sistantship from the Atomic Energy Commission, Contract No. AT(ll-1)-1049, through the Research Foundation is appreciated. Research done during the term of the latter assistantship provided material for this thesis. iii TABLE OF CONTENTS Chapter Page I. INTRODUCTION. 1 II. HISTORICAL, , 3 III. INTRODUCTION TO THE EXPERIMENTAL WORK 14 Objectives and Plan of the Study. -
![Chemistry 20 - Unit 2 - Ph and Poh Notes Name: ______+ - [H3O ] and [OH ] ](https://docslib.b-cdn.net/cover/7401/chemistry-20-unit-2-ph-and-poh-notes-name-h3o-and-oh-487401.webp)
Chemistry 20 - Unit 2 - Ph and Poh Notes Name: ______+ - [H3O ] and [OH ]
Chemistry 20 - Unit 2 - pH and pOH Notes Name: _________________________________________ + - [H3O ] and [OH ] + - + - In water both H3O and OH exist H2O + H2O ⇌ H3O + OH + - -14 These concentrations exist in a balanced relationship [H3O ][OH ] = 1.00 x 10 + - This relationship is inversely related ↑ [H3O ] = ↓ [OH ] Example 1: -4 What is the hydroxide ion concentration in a solution with a hydronium ion concentration of 2.59 x 10 M ? + - -14 [H3O ][OH ] = 1.00 x 10 −14 − 1.00×10 [OH ] = + [H3O ] −14 [OH−] = 1.00×10 = 3.86 × 10−11 2.59×10−4 Example 2: If 2.50 g of NaOH was dissolved in water to produce 500mL of solution, what would be the concentration of hydronium and hydroxide ions in solution? + - NaOH ⟶ Na + OH − mol 1 mol 1 [OH ] L = 2.50 g × 40 g × 0.500 L = 0.125 M + - -14 [H3O ][OH ] = 1.00 x 10 −14 −14 [H O+] = 1.00×10 = 1.00×10 = 8.0 × 10−14M 3 [OH−] 1.25 pH Review + pH = − log[H3O ] + −pH [H3O ] = 10 Example 3: What is the pH of a solution of 0.159 M HCl? + - HCl ⟶ H + Cl + pH = − log[H3O ] pH = − log[0.159 M] pH = 0.799 Example 4: What is the hydronium and hydroxide ion concentration of a solution with a pH of 2.42? + −pH −2.42 [H3O ] = 10 = 10 = 0.0038 M + - -14 [H3O ][OH ] = 1.00 x 10 −14 −14 − 1.00×10 1.00×10 −12 [OH ] = + = = 2.63 × 10 M [H3O ] 0.0038 M pOH - Power of OH Since: + - -14 [H3O ][OH ] = 1.00 x 10 Can take the “log” of both sides… pOH = − log[OH−] + - -14 -log([H3O ][OH ]) = -log(1.00 x 10 ) − −pOH [OH ] = 10 + - + - And -log([H3O ][OH ]) = -log[H3O ] + -log[OH ] Therefore… pH + pOH = 14 Example 5: A solution has a pH of 5.750. -

Broensted Acids and Bases: They Are Not Substances but Molecules Or Ions!
AJCE, 2014, 4(4), ISSN 2227-5835 BROENSTED ACIDS AND BASES: THEY ARE NOT SUBSTANCES BUT MOLECULES OR IONS! Hans-Dieter Barke Institute of Chemistry Didactics, University of Muenster, Germany [email protected] Nina Harsch Centre for Teacher Education, University of Muenster, Germany [email protected] ABSTRACT For chemistry education we are discussing mainly two concepts of acids and bases: theories of Arrhenius and Broensted. For the first theory, dissociation into ions is generally discussed: acidic solutions contain H +(aq) ions, alkaline solutions contain OH -(aq) ions. This theory therefore deals with substances, which are referred toas acids and bases – it would be even better to take the logical names "acidic and alkaline solutions". If both solutions are mixed + - in equivalent quantities, the H (aq) ions react with OH (aq) ions to form H 2O molecules, while the other ions remain in solution (in exceptional cases an insoluble salt may precipitate: sulfuric acid solution reacts with barium chloride solution to solid white barium sulfate and water). The Broensted theory defines proton transfer: a molecule or an ion transfers a proton to another molecule or ion, two conjugated acid-base pairs are involved. Thus, Broensted acids and bases are no more substances, but individual types of particles. Due to the autoionization of H 2O molecules (not "autoionization of water"), the following equilibrium exists: + - H2O + H 2O D H3O (aq) + OH (aq) + Through this protolysis it is more advantageous to argue rather with H 3O (aq) ions than with H +(aq) ions. In this theory there are still ampholyte particles which react as acid or as base - particles depending on the reaction partners: H 2O molecules, NH 3molecules , HSO 4 ions – water, ammonia or sodium hydrogen sulfate cannot be regarded asampholytes. -

An Organic/Inorganic Electrode-Based Hydronium-Ion Battery ✉ Zhaowei Guo1, Jianhang Huang1, Xiaoli Dong 1, Yongyao Xia1, Lei Yan1, Zhuo Wang1 & Yonggang Wang 1
ARTICLE https://doi.org/10.1038/s41467-020-14748-5 OPEN An organic/inorganic electrode-based hydronium-ion battery ✉ Zhaowei Guo1, Jianhang Huang1, Xiaoli Dong 1, Yongyao Xia1, Lei Yan1, Zhuo Wang1 & Yonggang Wang 1 Hydronium-ion batteries are regarded as one of the most promising energy technologies as next-generation power sources, benefiting from their cost effectivity and sustainability merits. Herein, we propose a hydronium-ion battery which is based on an organic pyrene- 1234567890():,; 4,5,9,10-tetraone anode and an inorganic MnO2@graphite felt cathode in an acid electrolyte. 2+ Its operation involves a quinone/hydroquinone redox reaction on anode and a MnO2/Mn + conversion reaction on cathode, in parallel with the transfer of H3O between two electrodes. The distinct operation mechanism affords this hydronium-ion battery an energy density up to 132.6 Wh kg−1 and a supercapacitor-comparable power density of 30.8 kW kg−1, along with a long-term cycling life over 5000 cycles. Furthermore, surprisingly, this hydronium-ion battery works well even with a frozen electrolyte under −40 °C, and superior rate performance and cycle stability remain at −70 °C. 1 Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Institute of New Energy, iChEM (Collaborative ✉ Innovation Centre of Chemistry for Energy Materials), Fudan University, Shanghai 200433, China. email: [email protected] NATURE COMMUNICATIONS | (2020) 11:959 | https://doi.org/10.1038/s41467-020-14748-5 | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-020-14748-5 ithium ion batteries (LIBs) using organic electrolytes have 2 e– 2 e– been extensively used to power various portable electronics L Mn2+ and electric vehicles (EVs) because of their desirable elec- PTO Cathode 1–9 +2 H O+ +4 H O+ trochemical performance . -

The Nature of the H3O Hydronium Ion in Benzene and Chlorinated Hydrocarbon Solvents
Published on Web 01/25/2006 + The Nature of the H3O Hydronium Ion in Benzene and Chlorinated Hydrocarbon Solvents. Conditions of Existence and Reinterpretation of Infrared Data Evgenii S. Stoyanov,* Kee-Chan Kim, and Christopher A. Reed* Contribution from the Department of Chemistry, UniVersity of California, RiVerside, California 92521 Received July 28, 2005; E-mail: [email protected] + Abstract: Salts of the C3v symmetric hydronium ion, H3O , have been obtained in the weakly basic solvents benzene, dichloromethane, and 1,2-dichloroethane. This is made possible by using carborane counterions - of the type CHB11R5X6 (R ) H, Me, Cl; X ) Cl, Br, I) because they combine the three required properties of a suitable counterion: very low basicity, low polarizability, and high chemical stability. The existence of + the H3O ion requires the formation of three more-or-less equivalent, medium-to-strong H-bonds with solvent - or anion bases. With the least basic anions such as CHB11Cl11 , IR spectroscopy indicates that C3v symmetric + trisolvates of formulation [H3O ‚3Solv] are formed with chlorocarbon solvents and benzene, the latter with the formation of π bonds. When the solvents and anions have comparable basicity, contact ion pairs of the + type [H3O ‚nSolv‚Carborane] are formed and close to C3v symmetry is retained. The conditions for the + existence of the H3O ion are much more exacting than previously appreciated. Outside of the range of solvent basicity bounded at the lower end by dichloromethane and the upper end by tributyl phosphate, and with anions that do not meet the stringent requirements of weak basicity, low polarizability of high + chemical stability, lower symmetry species are formed. -

Ch 17A Acid-Base Practice Problems
1 General Chemistry II Jasperse Acid-Base Chemistry. Extra Practice Problems General Types/Groups of problems: Conceptual Questions. Acids, Bases, and p1 Kb and pKb, Base Strength, and using Kb or p7-10 - + Conjugates, Miscellaneous pKb to Calculate [OH ], pOH, pH, and/or [H ] Recognizing Strong versus Weak Acids; p3 Recognizing Acid/Base Properties when p11 Recognizing Basic versus Nonbasic Ionics are Dissolved in Water pH Calculations; Relationships between pH and pOH p4 Answers p12 Ka: Sense + Calculations. Using Ka or pKa to Calculate p5 + [H ] and/or pH; using pH to calculate Ka or pKa Conceptual Questions. Acids, Bases, and Conjugates, Miscellaneous 1. In the Brønsted–Lowry definition of acids and bases, an acid __________ a. is a proton donor. d. breaks stable hydrogen bonds. b. is a proton acceptor. e. corrodes metals. c. forms stable hydrogen bonds. 2. In the Brønsted–Lowry definition of acids and bases, a base __________ a. is a proton donor. d. breaks stable hydrogen bonds. b. is a proton acceptor. e. corrodes metals. c. forms stable hydrogen bonds. 3. In the following reaction in aqueous solution, the acid reactant is __________ and its conjugate base product is __________. – + CH3COOH + NH3 CH3COO + NH4 – + a. CH3COOH; CH3COO d. NH3; NH4 + + b. CH3COOH; NH4 e. CH3COOH; H3O – c. NH3; CH3COO 4. In the following reaction in aqueous solution, the acid reactant is __________, and its conjugate base product is __________. – + 2– CH3NH2 + HSO4 CH3NH3 + SO4 + – 2– a. CH3NH2; CH3NH3 d. HSO4 ; SO4 2– – b. CH3NH2; SO4 e. HSO4 ; H3O+ – + c. HSO4 ; CH3NH3 2– 5. Which of the following is the conjugate acid of the hydrogen phosphate ion, HPO4 ? 3– a. -

Nacl + H2SO4 Nahso 4 +
SODIUM HYDROGEN SULFATE Chemical and Technical Assessment (CTA) Prepared by Madduri V. Rao, Ph.D. 1. Summary At the seventy-first meeting of JECFA, the Committee evaluated the safety, dietary intake and revision of specifications of sodium hydrogen sulfate (NaHSO4) at the request of the Codex Committee on Food Additives for its use as an acidifier. The Committee, at its sixty-eighth meeting (FAO/WHO, 2007), established specifications for sodium hydrogen sulfate for use in the preparation of acidified sodium chlorite, an antimicrobial washing solution. Sodium hydrogen sulfate, when added to food products containing water, ionizes to sodium ions, hydrogen ions, and sulfate ions. Since Acceptable Daily Intakes (ADIs) have been previously established for these ions, sodium hydrogen sulfate was not evaluated at the sixty-eighth meeting of the Committee. This document summarizes chemical and technical information on sodium hydrogen sulfate, partly based on information submitted by Jones- Hamilton Co.1. 2. Description Sodium hydrogen sulfate (NaHSO4, CAS No. 7681-38-1) is a colourless crystalline compound which is freely soluble in water. Sodium hydrogen sulfate, when added to food products containing water, ionizes to sodium ions, hydrogen ions, and sulfate ions. In addition to its use as an acidifier in foods (including its use in the preparation of acidified sodium chlorite), it also is used as an acidifier in pet foods, for pH control in swimming pools and SPAs, for ammonia control in livestock operations, and as an acid-type cleaner for commercial purposes. 3. Methods of Manufacture Sodium chloride and sulfuric acid are the starting materials in the manufacture of sodium hydrogen sulfate. -

Secondary Sulfate Minerals Associated with Acid Drainage in the Eastern US: Recycling of Metals and Acidity in Surficial Environments
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Geochemistry of Sulfate Minerals: A Tribute to Robert O. Rye US Geological Survey 2005 Secondary sulfate minerals associated with acid drainage in the eastern US: recycling of metals and acidity in surficial environments J.M. Hammarstrom U.S. Geological Survey R.R. Seal II U.S. Geological Survey A.L. Meier U.S. Geological Survey J.M. Kornfeld Dartmouth College Follow this and additional works at: https://digitalcommons.unl.edu/usgsrye Part of the Geochemistry Commons Hammarstrom, J.M.; Seal, R.R. II; Meier, A.L.; and Kornfeld, J.M., "Secondary sulfate minerals associated with acid drainage in the eastern US: recycling of metals and acidity in surficial environments" (2005). Geochemistry of Sulfate Minerals: A Tribute to Robert O. Rye. 2. https://digitalcommons.unl.edu/usgsrye/2 This Article is brought to you for free and open access by the US Geological Survey at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Geochemistry of Sulfate Minerals: A Tribute to Robert O. Rye by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Chemical Geology 215 (2005) 407–431 www.elsevier.com/locate/chemgeo Secondary sulfate minerals associated with acid drainage in the eastern US: recycling of metals and acidity in surficial environments J.M. Hammarstroma,*, R.R. Seal IIa, A.L. Meierb, J.M. Kornfeldc aU.S. Geological Survey, 954 National Center, Reston, Virginia 20192, USA bU.S. Geological Survey, 973 Denver Federal Center, Denver, Colorado 80225, USA cDartmouth College, Dartmouth, New Hampshire, USA Accepted 1 June 2004 Abstract Weathering of metal-sulfide minerals produces suites of variably soluble efflorescent sulfate salts at a number of localities in the eastern United States. -

GRADES 6-12 DISTANCE LEARNING School Name Aledo High School
GRADES 6-12 DISTANCE LEARNING School Name Aledo High School Grade Level Chemistry - Mrs. Henyon Week of 4/20/20, 4/27/20, 5/4/20 *All assigned work due by Sunday at midnight on 5/10/2020 (SUBJECT AREA) Estimated Time to Complete: 6 hours Resources Needed: Computer (if applicable), Scientific calculator, Journal, pen/pencil, Color pencils Lesson Delivery (What do we want you to learn?): 1. Define acids and bases and distinguish between Arrhenius and Bronsted-Lowrey definitions. 2. Predict products in acid-base reactions that form water. 3. Define pH. 4. Calculate the pH of a solution using the hydrogen ion concentration. Engage and Practice (What do we want you to do?): Digital Students: 1. Watch Video #1 Acids and Bases titled “How to Name Acids”. Due 4/26/20 2. Complete the Classifying Map and Critical Writing in journal. Upload picture of assignments to Google Classroom Due 4/26/20 *these are found in your NOTES. 3. Watch Video #2 Acids and Bases titled “Acid and Base Definitions”. Due 4/26/20 4. Complete HW #1 in your journal. Upload a picture of answers to Google Classroom. Due 5/3/20 5. Watch Video #3 titled “Self-Ionization of Water and Calculating pH”. Due 5/3/20 6. Complete the pH Worksheet, questions #1-8. Upload a picture of your answers to Google Classroom. Due 5/10/20 7. Complete the Acids and Bases Lab using the pHet simulator. Upload a picture of your work or a saved document to Google Classroom. Due 5/10/20 8. -

Chapter 8: Water As a Resource Homework Assignment: from The
3/16/19 Chapter 8: Water as a resource Homework assignment: From the end of the chapter, questions 3,5,7,9,13,19,21,23,27,29,35,41,44,47,54,59,60. From where do we get potable (fit for consumption) drinking water? Surface water: from lakes, rivers, reservoirs Ground water: pumped from wells drilled into underground aquifers 1 3/16/19 What is a solution? A solution is a homogeneous mixture of uniform composition. Solutions are made of solvents and solutes. Substances capable of Substances dissolved in a dissolving other substances- solvent- usually present in the usually present in the greater lesser amount amount When water is the solvent, the solution is an aqueous solution. 2 3/16/19 Working with solutions: concentration terms (1) Parts per hundred (percent) 20 g of NaCl in 80 g of water is a 20% (w/w) NaCl solution Parts per million (ppm) (w/w) solution: 1 g solute 1 mg solute 1 mg solute 1 ppm = = = 1,000, 000 g water 1,000 g water 1 L water Parts per billion (ppb) (w/w) solution: 2 g Hg 2 ×10-6 g Hg 2 µg Hg 2 ppb Hg = = = 9 3 1 L H O 1×10 g H 2O 1×10 g H2O 2 Working with solutions: concentration terms (2) Molarity (M) = moles solute/liter of solution Consider a 1.0 M NaCl solution: [NaCl] = 1.0 M = 1.0 mol NaCl/L solution Because the molar mass of NaCl is 58.44 g, 1 liter of this solution would have 58.44 g NaCl enough water to give a total volume of 1.0 L. -
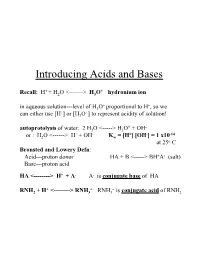
Introducing Acids and Bases
Introducing Acids and Bases + + Recall: H + H2O <-------> H3O hydronium ion + + in aqueous solution---level of H3O proportional to H , so we + + can either use [H ] or [H3O ] to represent acidity of solution! + - autoprotolysis of water: 2 H2O <-----> H3O + OH + - + - -14 or : H2O <------> H + OH Kw = [H ] [OH ] = 1 x10 at 25o C Bronsted and Lowery Defn: Acid---proton donor HA + B <-----> BH+A- (salt) Base---proton acid HA <--------> H+ + A- A- is conjugate base of HA + + + RNH2 + H <--------> RNH3 RNH3 is conjugate acid of RNH2 Consequences of Autoprotolysis rxn of Water: •can always find concentration of H+ or OH- if other species is known since in an aqueous solution---the autoprotolysis rxn is always in equilibrium!----product of [H+] [OH-] must equal 1 x10-14 + -3 - + -12 e.g.---if you know [H ] = 2 x 10 M; then [OH ] = Kw/ [H ] = 5 x 10 •in pure water---[H+] [OH-] = x2 = 10-14; Hence [H+] = [OH-] = 1 x 10-7 pH and [H+]; + + define pH = - log [H ] (we will see later that pH = -log aH ---but + + in dilute solutions---[H ] = aH ) if [H+] - 3.8 x 10-8 ; pH = - log(3.8 x 10-8) = 7.42 pH Scale pH = -log [H+] ; therefore: [H+] = 10-pH (can have pH values < 0 (negative #) and > 14--very strong acids/bases at very high concentrations) 0.1 M HCl strong acid--100% dissociation 0.001 M HCl • 0.00001 M HCl • What about 1x10-8 M HCl? pH=8 ?? – A dilute acid cannot be basic. We shall see--in a minute! pH for strong acid and strong base determined by moles of H+ or OH- generated by complete dissociation of these species; -3 + - 10 M HCl; HCl(aq)