Generation and Transmission of Inter-Lineage Recombinants in the SARS-Cov-2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DOT-LEM35 Laser Engraving Machine
Oil & Gas Petrochemical Power Renewable Energy Ship Building Building Services international Product Brochure Electrical, Instrumentation, Mechanical & Safety Labelling & Identification Solutions T: +44 (0)1582 703 703 E: [email protected] www.DOTgroup.net Contents Introduction to DOTgroup International . .2 DOTgroup International Project Portfolio . .3 DOTcard System DOTcable862, DOTcable864 & DOTcable458 Cable Markers & DOTvalve3 Valve Tags . .8 DOTcard2130 Core Idents and DOTsleeve Transparent Sleeves . .9 DOTcard System Printers, Ribbons and Consumables . .10 DOT-FCM Flexible Cable Marker / DOTshrink System DOT-FCM – Flexible Cable Markers . .12 DOTshrink – Heatshrink Tubing . .13 DOTwrap4x2 – Wraparound Self-adhesive Labels . .14 DOT-FCM / DOTshrink System Printer, Perforator / Cutter and Ribbon . .15 DOT-PHP Portable Heatshrink Printer & DOT-PHC Portable Heatshrink Cartridge . .16 DOT-LEM35 Laser Engraving System DOT-LEM35 Laser Engraving Machine . .18 DOT-LEM35 Laser Fume Extraction System & DOT-LEM35 Laptop . .19 DOT-SSCM8919 & DOT-SSCM8910 Stainless Steel Cable Markers . .20 DOT-SSDT26 & DOT-SSDT38 Stainless Steel Dog Tags . .20 Bespoke Stainless Steel Labels, Signs and Plates . .21 DOTlaser Flexible Engraving Laminate . .22 General Labelling & Identification Products, Bespoke Solutions & Accessories DOT-REL Rigid Engraving Laminate, Ball Chain & Connectors . .24 DOT-RELEM Rigid Engraving Laminate Engraving Machine & DOT-REL-S1 Saw . .25 DOT Nylon Cable Ties . .26 DOT Stainless Steel Cable Ties & Stainless Steel Cable Strap / Cushion Sleeve System . .27 DOT Pipeline Identification Tape . .28 DOT Manual Cable Markers & Carrier Strips . .29 DOT Manual Chevron Cut, Straight Cut & Open Style Core Idents and Applicators . .30 Fire, Safety, Hazardous Area & General Warning Signs . .32 DOT-SFC - Slim Fix Clips . .34 DOTlabel Software . .35 DOT Underground Cable Protection Covers & Underground Cable Protection Rolls . -

International Engineering Group Spie Matthew Hall
INTERNATIONAL ENGINEERING GROUP SPIE MATTHEW HALL CELEBRATES 160 YEARS OF BUSINESS WITH THE OPENING OF FOUR NEW OFFICES Submitted by: Beyond PR (Oxford) Tuesday, 7 October 2008 SPIE Matthew Hall, the leading multi technical and support services company, has opened four new facilities in Poyle, near Heathrow, Manchester, Glasgow and Cardiff. The Manchester and Glasgow offices are relocations to larger premises due to the expansion of its customer base. Cardiff is a brand new venture to allow SPIE Matthew Hall to better service its customers in Wales and build its business in the region. Heathrow will continue to support the airport sector as well as customers in the Thames Valley. 2008 sees the companies’ 160th commemoration having being founded by Matthew Hall in 1848. Established as a lead working and plumbing business the Matthew Hall brand was involved in prestigious projects from the outset carrying out engineering works on buildings such as Buckingham Palace, Windsor Castle and the Natural History Museum. SPIE Matthew Hall continues in the tradition of its market leading heritage and has recently completed extensive mechanical and electrical engineering installations at Heathrow’s new Terminal 5. Other recent multi technical projects have included St Pancras International, Shell Centre and the current Savoy Hotel refurbishment in London. Kevin Morgan, Managing Director of SPIE Matthew Hall said, “The opening of the new offices demonstrates SPIE Matthew Hall’s commitment to its customers in the UK. The Matthew Hall name is synonymous with quality and reliability and this year is the 160th commemoration of the Matthew Hall brand and it continues to go from strength to strength. -
-

The J. Paul Getty Trust 2007 Report the J
THE J. PAUL GETTY TRUST 2007 REPORT THE J. PAUL GETTY TRUST 2007 REPORT 4 Message from the Chair 7 Foreword 10 The J. Paul Getty Museum Acquisitions Exhibitions Scholars Councils Patrons Docents & Volunteers 26 The Getty Research Institute Acquisitions Exhibitions Scholars Council 38 The Getty Conservation Institute Projects Scholars 48 The Getty Foundation Grants Awarded 64 Publications 66 Staff 73 Board of Trustees, Offi cers & Directors 74 Financial Information e J. Paul Getty Trust The J. Paul Getty Trust is an international cultural and philanthropic institution that focuses on the visual arts in all their dimensions, recognizing their capacity to inspire and strengthen humanistic values. The Getty serves both the general public and a wide range of professional communities in Los Angeles and throughout the world. Through the work of the four Getty programs–the Museum, Research Institute, Conservation Institute, and Foundation–the Getty aims to further knowledge and nurture critical seeing through the growth and presentation of its collections and by advancing the understanding and preservation of the world’s artistic heritage. The Getty pursues this mission with the conviction that cultural awareness, creativity, and aesthetic enjoyment are essential to a vital and civil society. e J. Paul Getty Trust 3 Message from the Chair e 2007 fi scal year has been a time of progress and accomplishment at the J. Paul Getty Trust. Each of the Getty’s four programs has advanced signifi cant initiatives. Agreements have been reached on diffi cult antiquities claims, and the challenges of two and three years ago have been addressed by the implementation of new policies ensuring the highest standards of governance. -

July-2013.Pdf
HALL OF FAME: VIRGIL HILL’S UNLIKELY JOURNEY TO CANASTOTA 168 POUNDS 147 POUNDS 135 POUNDS OUR IN-DEPTH AND EXCLUSIVE DIVISION-BY-DIVISION ANALYSIS FRESH START NEW USA BOXING BOSS OPTIMISTIC AMERICANS WILL REBOUND HBO VS. SHOWTIME BATTLE OF NETWORKS HEATS UP WITH MAYWEATHER’S DEFECTION HE’S BACK DAVID HAYE STILL COMMANDS ATTENTION AS HE PLOTS HIS FUTURE JULY 2013 JULY SAFETY FIRST $8.95 BRAIN HEALTH STUDY AIMS TO EDUCATE PROFESSIONAL FIGHTERS CONTENTS JULY 2013 FEATURES DEPARTMENTS 4 | RINGSIDE 5 | OPENING SHOTS 10 | COME OUT WRITING 13 | ROLL WITH THE PUNCHES Jabs and Straight Writes by Thomas Hauser 18 | NEW FACES: FRANKIE GOMEZ By Mike Coppinger 21 | RING CARD GIRL 25 | READY TO GRUMBLE By David Greisman 28 | OUTSIDE THE ROPES 31 | SWEET SCIENCE By Scott LaFee 34 | WOMEN’S BOXING By Tom Gerbasi 36 | RING RATINGS PACKAGE 78 | BEST I’VE FACED: VIRGIL HILL By Anson Wainwright 100 | LETTERS FROM EUROPE By Gareth A Davies 104 | RINGSIDE REPORTS 84 108 | WORLDWIDE RESULTS 110 | COMING UP COVER STORY 84 FRESH START 112 | FROM THE ARCHIVE 44 STATE OF THE GAME NEW USA BOXING PRESIDENT THE RING’S EXCLUSIVE DIVISION-BY- BURSTING WITH OPTIMISM 114 | AT THE FIGHTS DIVISION ANALYSIS By Bernard Fernandez By Don Stradley 90 IMPACT OF PUNCHES AT RINGTV.COM CANELO WINS PRAISE 68 HBO VS. SHOWTIME BRAIN STUDY AIMS TO EDUCATE AND Saul “Canelo” Alvarez BATTLE OF THE BOXING PROTECT BOXERS earned the RING title and NETWORKS HEATS UP By Gordon Marino grudging respect with his By Tim Smith victory over Austin Trout. Read RingTV.com Editor 72 VIRGIL HILL Doug Fischer’s column. -

View EPIC's Renewed Petition, Sent on July 26, 2013. (PDF)
July 26, 2013 General Keith B. Alexander Director, National Security Agency 9800 Savage Road Fort Meade, MD 20755 Secretary Chuck Hagel Department of Defense 1400 Defense Pentagon Washington, DC 20301 Re: Petition for NSA to Conduct a Public Rulemaking on its Monitoring and Collection of Communications Traffic within the United States Dear General Alexander and Secretary Hagel: The undersigned individuals and organizations, concerned about the rule of law and the protection of Constitutional freedoms, hereby petition the National Security Agency to conduct a public rulemaking on the agency's monitoring and collection of communications traffic within the United States. 5 U.S.C. § 553(e). We believe that the NSA’s collection of domestic communications contravenes the First and Fourth Amendments to the United States Constitution, and violates several federal privacy laws, including the Privacy Act of 1974, and the Foreign Intelligence Surveillance Act of 1978 as amended. The NSA’s collection of solely domestic communications, which has been acknowledged by the President, the Director of National Intelligence, and the Chair and Ranking Member of the Senate Select Committee on Intelligence, also constitutes a legislative rule that “substantively affects the public to a degree sufficient to implicate the policy interests animating notice-and- comment rulemaking” under the Administrative Procedure Act. EPIC v. DHS, 653 F.3d 1, 6 (D.C. Cir. 2011). Accordingly, the NSA's collection of domestic communications, absent the opportunity for public comment, is unlawful. We hereby petition the National Security Agency, a component of the Department of Defense, for relief. We ask the NSA to immediately suspend collection of solely domestic communications pending the completion of a public rulemaking as required by law. -

Political Traditions and Scottish Devolution
Political Traditions and Scottish Devolution by Matthew Hall A thesis submitted to The University of Birmingham for the Degree of DOCTOR OF PHILOSOPHY Department of Political Science and International Studies School of Government and Society The University of Birmingham April 2009 1 Abstract This thesis seeks to develop a conception of the political traditions operating in the UK and then apply it to the development of Scottish Devolution. I argue that the concept of tradition has been under-valued and theorised in social science and that the notion of political traditions has heuristic value when applied to British politics. Discussion of a distinctive British Political Tradition has been kept to the margins in explanations of the British political system with only a few authors seeking to explore the ideational underpinnings of the institutions and process of British government and the Westminster Model. The recent work of Bevir and Rhodes has raised the profile of political traditions, however I contend that their conceptualisation is flawed and thus, heuristically limited. I argue that we can identify a dominant political tradition, the British Political Tradition, which has decisively influenced the nature and conduct of British political life over time. This tradition expresses and facilitates the ideas and interests of dominant socio-economic groups in UK society. However it has not gone uncontested. We can also identify the existence of competing political traditions which challenge aspects or the entirety of, the British Political Tradition. Although competing political traditions resonate asymmetrically, it is through the process of conflict and contestation that changes in the British political system can be explained. -

View the Conference Programme
The PROTAX has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 787098 Horizon 2020 project BRINGING STAKEHOLDERS TOGETHER Addressing Human and Legal Factors in Tax Compliance and Enforcement in the EU and Beyond INTERNATIONAL CONFERENCE 7-8 July 2021 protax-project.eu OVERVIEW The International Conference on “Bringing Stakeholders Together – Addressing Human and Legal Factors in Tax Compliance and Enforcement in the EU and Beyond” will be held on 7-8 July 2021, marking the completion of the PROTAX project. PROTAX (“New Methods to Prevent, Investigate and Mitigate Corruption and Tax Crimes in the EU”) is an EU-funded H2020 research project (2018-2021) which has generated policy guidelines and toolkits to harmonise the treatment of tax crime across the EU Member States. The main idea behind this international conference is to bring together leading experts in preventing and countering tax crimes, good tax governance and integrity in businesses to share and debate the novel findings by PROTAX. The PROTAX Conference panels will focus on: • Tax system design and crime prevention in the EU and beyond; • International and EU responses to serious and organised tax crimes; • Challenges in cross-border investigations for national gatekeepers: • Illicit financial flows and tax evasion; • Institutional and fiscal corruption; • Human factors in tax compliance and enforcement; • The role of professionals in tax compliance; • Cutting-edge strategies and toolkits to prevent and counter tax crimes in the EU. Contributions will be made by leading academics, representatives from international institutions, policymakers, tax crime investigators, public prosecutors, judges, tax and customs officers, asset recovery specialists, financial intelligence unit officers, anti-money laundering and anti-corruption experts, tax advisors, accountants and lawyers. -

SPIE MATTHEW HALL LAUNCHES MOBILE SUPPORT SERVICE to FURTHER ENHANCE ITS FM OFFERING Submitted By: Beyond PR (Oxford) Wednesday, 11 February 2009
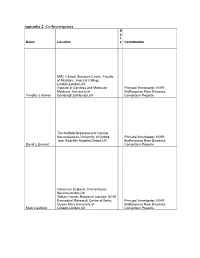
SPIE MATTHEW HALL LAUNCHES MOBILE SUPPORT SERVICE TO FURTHER ENHANCE ITS FM OFFERING Submitted by: Beyond PR (Oxford) Wednesday, 11 February 2009 SPIE Matthew Hall (http://www.spiematthewhall.com), the leading multi technical and support services company, has invested in the further expansion of its Facilities Management offering with the launch of its Mobile Support Service. The Mobile Support Service operates through a national network of offices located within key cities. Providing maintenance, repair and installation services for all Mechanical, Electrical and associated building services, together with the existing Fire Engineering Test and Inspection business, this focussed mobile service has been established to support and complement the SPIE Matthew Hall’s Facilities Services business. Recognising the clients’ needs for a responsive, personal and effective service, SPIE Matthew Hall (http://www.spiematthewhall.com) has opened a new 24 hour call centre which co-ordinates the mobile operation and offers telephone support to its customers and technicians. The national call centre will ensure a fast and friendly response to all enquiries along with the planning and progress of works necessary to ensure jobs are completed on time and within budget as well as keeping clients fully informed. David Gannon, General Manager of SPIE Matthew Hall’s Mobile Service explains: “The SPIE Matthew Hall business has proven experience within Facilities Management and the Mobile Support Service is a further extension of this capability. Our technicians are located throughout the UK and will provide an efficient and effective response to our customers’ needs. By maintaining a defined region of clients, our technicians quickly become familiar with customers requirements, expectations and on site procedures. -

Herefordcathedralitw 0000000
1 2 5 4 3 7 6 9 8 OLD HEREFORDIANS’ CLUB NEWSLETTER 2009 OH News Debut Novel for Matthew Hall TV screenwriter Matthew Hall (OH) has recently From the President Elect published his first novel, The Coroner. After three years at the helm of Drawing on his own experiences as a criminal barrister, the Old Herefordians’ Club the this debut novel is set in the Severn Vale and is the first of time has come for Mark Ellis to a series featuring a small-town lawyer turned coroner, step down. As incoming President, Jenny Cooper. Jenny, a complex character with her own before all else I really have to problems, exposes some controversial areas of the thank Mark for all his time criminal justice system. The Coroner has been critically and considerable hard acclaimed by other crime writers: ‘M R Hall has created a work in bringing the club wonderful heroine in a genre we haven't seen before.' – to where it is today and I look forward to his Lynda La Plante. Matthew’s second novel in the series, continuing support as a The Disappeared, is due to be published in January 2010. committee member. For many years Matthew has had a highly successful I left HCS in 2000 and I am a television screenwriting career and his TV credits include: relative newcomer to the OH Kavanagh QC (ITV) – Men of Substance; A Sense of Loss; Blood Committee, by which I mean there are members of the Money; Wing and a Prayer (C5) – BAFTA nominated original committee who have been involved with the Old series in 14 episodes; Dalziel and Pascoe (BBC1) – Above the Herefordians since before I was born! Law; Walls of Silence; Scarlet Pimpernel (BBC1) - Ennui; Loving The OH Committee meets regularly throughout the Yo u (ITV); New Street Law; year. -

Magnificent Seven Preview
Magnificent Seven preview This Saturday several of the best fighters in Britain appear on a bumper Frank Warren promotion called “The Magnificent Seven” Though a few weeks back we had Darren Barker & Ryan Rhodes pull of there respective fights some good work behind the scene’s saw both fights saved with replacements stepping in. Here we look at the fights that make up an exciting night of Boxing that kicks off at 6pm on SKY PPV. Nathan Cleverly –V- Karo Murat WBO Light Heavyweight Title Eliminator. http://www.15rounds.com/q-a-with-nathan-cleverly-031610/ http://www.15rounds.com/q-a-with-karo-murat-072710/ Hometown; Cefn Forest. Wales Kitzingen, Germany Record; 19-0(9) 22-0(13) Rounds boxed; 101 121 KO% 47 59 Age; 23 27 Height; 6’3 5’10 Rankings; The Ring Cleverly 8 Murat 6, Neither fighter rated in the top 15 by WBC or WBA. IBF Cleverly 4 & Murat 11 & WBO Cleverly 2 & Murat 3. Betting 1/4 11/4 This fight is see’s both guys fighting the best fighter they have to date. Cleverly will look to dominate with the jab and put Murat on the back foot using his height and reach advantages. By the mid rounds Murat will become marked up and frustrated at being repeatedly caught trying to get inside. To Murat’s credit he’ll continue to try, while Cleverly will build up the points on way to a comfortable decision. Enzo Maccarinelli –V- Alexander Frenkel European Cruiserweight Title http://www.15rounds.com/q-a-with-enzo-maccarinelli-082610/ http://www.15rounds.com/q-a-with-alexander-frankel/ Hometown; Swansea, Wales Wurzberg, Germany Record; 32-4(25) 22-0(17) Rounds Boxed; 128 74 KO% 69 77 Age; 30 25 Height; 6’4 6’1 Rankings; Neither fighter ranked in the Ring top 10; WBC Maccarinelli 8 Frenkel 7; WBA Maccarinelli 3 Frenkel 2; IBF Maccarinelli 11 Frenkel 6 & WBO Maccarinelli unranked Frenkel 12.