Characterization of Hermetia Illucens (Diptera: Stratiomyidae) Midgut
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Silvia Wojczewski EDUCATION EMPLOYMENT
Silvia Wojczewski Wiedner Hauptstrasse 130/12 1050 Vienna AUSTRIA +43 650 4146789 [email protected] http://www.researchgate.net/profile/Silvia_Wojczewski Nationalities: German and French EDUCATION 2011 Mag. phil., Social- and Cultural Anthropology, University of Vienna, Austria 2009 BSc. Environment and Bio Resource Management, University of Applied Life Sciences, Vienna, Austria EMPLOYMENT 02/2016-2021 PhD candidate and teaching assistant (supervisor: Anne-Christine Trémon, Co- supervisor: David Picard) at the University of Lausanne, Institute for Geography and Sustainability, Anthropology of Travel and Tourism http://igd.unil.ch/silviawojczewski/en/presentation/ Associate member of the LACS – Laboratoire d’anthropologie sociale et culturelle, Université de Lausanne 10/2015 External lecturer, Medical anthropology, Medical University, Vienna 03/2013- 09/2015 Research assistant EU-FP7-project HURAPRIM Human Resources for Primary Health Care in Africa/ Migration of health care workers (http://www.huraprim.ugent.be/drupal/), APRES about Antibiotic resistance in Europe, QUALICOPC about General Medicine in Europe and COCO- Common Cold-Project in Europe, Unit Ethnomedicine and International Health, Medical University, Vienna, Austria 08/2012 Project assistant quantitative methods for Dr. Gerhild Trübswasser, intercultural project from the University of Klagenfurt, Austria 02/2012- 06/2012 Project assistant part-time qualitative methods at the Unit Ethnomedicine and International Health, Medical University, Vienna 05/2011- 12/2013 Translator -

Lausanne / Switzerland Energy-From-Waste Plant
Lausanne / Switzerland General project data Owner TRIDEL SA Energy-from-Waste Plant Start of operation 2006 Total investment CHF 360 million Scope of Hitachi Zosen Inova AG Combustion, flue gas treatment, residue treatment Plant design Hitachi Zosen Inova AG Technical data Annual capacity 160,000 t/a Number of trains 2 Throughput per train 10 t/h (nom), 12.5 t/h (max) Calorific value of waste 14.4 MJ/kg (nom), 7.2–18.0 MJ/kg (min./max.) Thermal capacity per train 40 MW Waste type Municipal solid and commercial waste Special waste fractions Hospital waste, sewage sludge Waste delivery Waste pit capacity 10,000 m3 Bulk waste shredding Shredder Combustion system Grate type Hitachi Zosen Inova grate Grate design 2 rows with 4 zones per row Grate size Length: 8.5 m, width: 5.2 m Grate cooling First two zones water-cooled (Aquaroll®) Boiler Type Four-pass boiler, horizontal with external economiser Steam quantity per train 48.3 t/h Steam pressure 50 bar Steam temperature 400°C Flue gas outlet temperature 160°C (end of operations campaign) after external economiser Flue gas treatment Concept Electrostatic precipitator, wet scrubber, SCR, wastewater treatment Flue gas volume per train 63,000 m3/h (at standard conditions) Energy recovery Type Extraction condensation turbine Electric power output 20 MW (max. generator output) Heat generation 50 MW Residues Bottom ash 37,600 t/a (including treated fly ash) Zinc concentrate 1,400 t/a from fly ash scrubbing Special features Commodity recycling Acid fly ash washing with metal recovery Recovery of 180 t/a zinc and 1.7 t/a mercury Replacement 2 x 12.5 t/h, 40 MW Lausanne / Switzerland Energy-from-Waste Plant Tridel – environmentally-friendly thanks to maximized metals recovery and minimal emissions High energy efficiency, low emissions, virtually no visible plumes of steam: The new waste treatment plant in Lausanne, in the Canton of Vaud (CH), meets all the standards for a facility located near this urban recreational area. -

Luis Franjo García February 2017
LUIS FRANJO GARCÍA FEBRUARY 2017 CONTACT INFORMATION College of Management Phone Contact: Swiss Federal Institute of Technology Lausanne (EPFL) Office: +41 21 693 00 76 Station 5 Cell Phone 1: +41 78 825 56 93 CH – 1015 Lausanne, Switzerland Cell Phone 2: +34 69 988 86 45 Email: [email protected] URL: https://sites.google.com/site/luisfranjogarcia/home CURRENT POSITION October 2013 to present Postdoc Fellow, Chair of International Finance, Swiss Federal Institute of Technology Lausanne (EPFL). EDUCATION October 2013 Ph.D. in Economics, Universidad Carlos III de Madrid. MSc in Economics, Universidad Carlos III de Madrid. BA in Economics, Universidad Carlos III de Madrid. RESEARCH INTERESTS Primary Fields Macroeconomics, International Macroeconomics, Housing. Secondary Fields Growth, Consumption and Savings. VISITING POSITIONS 2009 (Feb – Jun) Instituto Tecnológico Autónomo de México (ITAM). WORK EXPERIENCE 2006-2008 Department of International Economics, Bank of Spain. Research Assistant. 1 TEACHING EXPERIENCE Instructor: 2015 - Global Business Environment, Master in Management, Technology and Entrepreneurship, École Polytechnique Fédérale de Lausanne (EPFL). - Macrofinance, Master in Financial Engineering, École Polytechnique Fédérale de Lausanne (EPFL). 2013 Recursive Methods in Macroeconomics, Ph.D. course, École Polytechnique Fédérale de Lausanne (EPFL). Teaching Assistant: 2013-2016 Global Business Environment, Master in Management, Technology and Entrepreneurship, École Polytechnique Fédérale de Lausanne (EPFL). 2012 Principles of Economics, undergraduate course, Universidad Carlos III de Madrid. 2010-2012 Microeconomics, undergraduate course, Universidad Carlos III de Madrid. 2010 Macroeconomics IV, undergraduate course, Universidad Carlos III de Madrid. 2010 Macroeconomics, undergraduate course, Universidad Carlos III de Madrid. 2009 Public Economy II, undergraduate course, Universidad Carlos III de Madrid. -

Paris to Brussels
( BRUSSELS (2) ( LuxemFrbaonukfrug rt PARIS (2) HEIDELBERG (1) ROTHENB Versailles Dinkelsbe STRASBOURG (1) Dachau MU TGV Neuschwanstein LUCERNE (2) Lausanne Lausanne Gruyeres Alpine INNSBR MoInnttererluaxken Train GENEVA (2) Montreux GENEVA (2) 9 DAYS What’s Included • Round-trip airfare • 7 nights in three & four-star hotels • Full-time CHA Tour Director • Breakfast & dinner daily • On-tour transportation by private motorcoach & train • Guided sightseeing & walking tours • Visits shown in italics in itinerary Paris to Brussels Day 1: Departure from the USA how traditional Swiss cheese is made, and 2018 TOUR PRICES stop in Lausanne, a thriving university town Day 2: Paris Welcome to Paris where your Tour with lovely views of Lake Geneva and the Alps. Oct 1- Feb 1- Mar 18- May 16- Jan 31 Mar 17 May 15 Sept 30 Director greets you and escorts you via motor - Drive back New York 2239 2389 2749 2929 coach to your hotel. Later, get better acquainted Day 6: Geneva-Strasbourg Boston 2279 2439 2809 2989 with the “City of Lights” on a CHA Walking Tour. into France as you head for Strasbourg in Alsace. Philadelphia 2299 2449 2799 2999 Named the “City of Roads” because of the meet - Syracuse/Buffalo 2379 2529 2869 3069 Day 3: Paris-Versailles-Paris The treasures ing point of highways, railways and waterways Pittsburgh 2319 2469 2829 3019 of Paris are yours to discover on your morning Washington/Baltimo r e 2319 2469 2819 3019 linking the Rhineland and the Mediterranean, Norfolk 2389 2539 2889 3079 tour with your expert Parisian guide. See the Strasbourg is the seat of the Council of Europe Richmond/Roanoke 2419 2569 2919 3119 Place de l’Opera, the Place de la Concorde, the and a modern metropolis with a thriving univer - Detroit 2359 2509 2859 3069 Arc de Triomphe, the Champs-Elysees, and the sity. -

Ultrasonic Vibration Assisted Manufacturing of High-Performance Materials
Ultrasonic vibration assisted manufacturing of high-performance materials by Fuda Ning, B.S., M.S. A Dissertation In Industrial, Manufacturing, and Systems Engineering Submitted to the Graduate Faculty of Texas Tech University in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY Approved Dr. Weilong Cong Chair of Committee Dr. Hong-Chao Zhang Dr. Golden Kumar Dr. George Zhuo Tan Dr. Wei Li (Dean’s Representative) Mark Sheridan Dean of the Graduate School May, 2018 Copyright 2018, Fuda Ning Texas Tech University, Fuda Ning, May 2018 To My Family and Fleeting Time. ii Texas Tech University, Fuda Ning, May 2018 ACKNOWLEDGMENTS First and foremost, I would like to express the deepest appreciation to my advisor, Dr. Weilong Cong, for his supervision and tremendous help throughout the past few years. He continually conveyed a rigorous attitude toward research and scholarship, which inspired me all the time. This dissertation would not have been completed without his professional guidance. I would like to thank my committee members, Dr. Hong-Chao Zhang, Dr. Golden Kumar, and Dr. George Zhuo Tan, for their valuable advice on my dissertation. I also want to thank Dr. Wei Li to serve as the Dean’s Representative for my defense. My sincere appreciation goes to the U.S. National Science Foundation for the financial support through award CMMI-1538381. I would like to extend my thanks to the Gradual School at Texas Tech University for the Doctoral Dissertation Completion Fellowship to enable me to devote full-time effort to finalizing this dissertation in the very last year of my Ph.D. -

Olivier J. Walther
Olivier J. Walther Department of Geography • University of Florida 3205 Turlington Hall • Gainesville, FL 32611 [email protected] • (352) 273-4739 @ojwalther • olivierwalther.net Revised: April 29, 2020 EDUCATION Ph.D. in Geography, University of Lausanne, Switzerland, 2006 Master of Advanced Studies in Development Studies, University of Geneva, 2004 Master of Arts in Geography, University of Lausanne, Switzerland, 2001 Bachelor of Arts, University of Lausanne, Switzerland, 1999 WORK EXPERIENCE Assistant Professor, Department of Geography, University of Florida, 2019 – Visiting Associate Professor, Center for African Studies, University of Florida, USA, 2017-19 Associate Professor, Center for Border Region Studies and Center for War Studies, Department of Political Science, University of Southern Denmark, Denmark, 2014 – Visiting Assistant Professor, Geography Department, Center for African Studies and Division of Global Affairs, Rutgers University, USA, 2013 Senior Researcher, Department of Geography, Centre for Population, Poverty and Public Policy Studies, Luxembourg, 2007-2012 Visiting Scholar, University of Basel, Switzerland, fall 2010 Research and Teaching Assistant, Institute of Geography, University of Lausanne, Switzerland, 2003-2006 RESEARCH AND TEACHING AREAS − Security and Conflict: Cross-border conflicts and terrorism in West Africa − International Trade: Regional integration in West Africa − Economic Geography: Cross-border city regions in Europe − Quantitative Methods: Social Network Analysis 1 RESEARCH PROJECTS Linking deforestation, urbanization, and agricultural expansion for land-use decisions in Ghana (2020-22) Co-Investigator (PI: Jasmeet Judge). Funded by NASA SERVIR-USAID ($ 660,000) Insecurity in the Sahel (2019-20) Principal Investigator. Funded by the OECD ($ 311,000) Cities and Borders (2017-18) Co-Principal Investigator. Funded by the OECD ($ 335,000) Gender and Markets: Empowering Women in West African Markets (2017) Co-Investigator. -
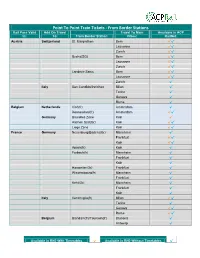
From Border Stations Rail Pass Valid Add on Travel Travel to Main Available in ACP In: To: from Border Station: Cities: Railnet: Austria Switzerland St
Point To Point Train Tickets - From Border Stations Rail Pass Valid Add On Travel Travel To Main Available in ACP In: To: From Border Station: Cities: RailNet: Austria Switzerland St. Margrethen Bern √√ Lausanne √√ Zurich √√ Buchs(SG) Bern √√ Lausanne √√ Zurich √√ Landeck-Zams Bern √√ Lausanne √√ Zurich √ Italy San Candido/Innichen Milan √ Torino √ Genova √ Rome √ Belgium Netherlands Visé(fr) Amsterdam √ Roosendaal(fr) Amsterdam √√ Germany Bruxelles Zone Koln √ Aachen Süd(Gr) Koln √√ Liege Zone Koln √√ France Germany Neuenburg(Baden)(Gr) Mannheim √ Frankfurt √√ Koln √√ Apach(fr) Koln √ Forbach(fr) Mannheim √ Frankfurt √ Koln √ Hanweiler(Gr) Frankfurt √ Wissembourg(fr) Mannheim √ Frankfurt √ Kehl(Gr) Mannheim √ Frankfurt √ Koln √ Italy Ventimiglia(fr) Milan √√ Torino √ Genova √√ Rome √√ Belgium Blandain(fr)/Tourcoin(fr) Brussels √ Antwerp √ Available in RN2 With Timetables √ Available in RN2 Without Timetables √ Point To Point Train Tickets - From Border Stations Rail Pass Valid Add On Travel Travel To Main Available in ACP In: To: From Border Station: Cities: RailNet: France Switzerland Le Châtelard(fr) Bern √ Lausanne √ Zurich √ Genève Bern √√ Lausanne √√ Zurich √√ Vallorbe(fr) Lausanne √ Basel SBB Bern √√ Lausanne √√ Zurich √√ Spain Port-Bou(fr) Barcelona √ Madrid √ Luxembourg Bettembourg(fr) Luxembourg √√ Germany Switzerland Konstanz Bern √√ Lausanne √√ Zurich √√ Basel Bad Bf Bern √√ Lausanne √√ Zurich √√ Waldshut Bern √√ Lausanne √√ Zurich √√ Belgium Aachen Süd(Gr) Brussels √ Liege Zone Brussels √√ Italy Switzerland Chiasso Zurich √ Iselle transito Bern √ Lausanne √ Zurich √ Austria San Candido/Innichen Innsbruck √ Salzburg √ Vienna √ Netherlands Belgium Visé(fr) Brussels √ Switzerland Germany Basel Bad Bf Stuttgart √√ Munich √√ Konstanz Stuttgart √√ Munich √√ Waldshut Stuttgart √√ Munich √√ Available in RN2 With Timetables √ Available in RN2 Without Timetables √ Point To Point Train Tickets - From Border Stations Rail Pass Valid Add On Travel Travel To Main Available in ACP In: To: From Border Station: Cities: RailNet: Switzerland Austria St. -

Expat City Ranking 2019 Introduction & Short Methodology
Expat City Ranking 2019 Introduction & Short Methodology Expat City Ranking To identify the best and worst cities for expats, survey respondents were asked to rate more than 25 different aspects of urban life abroad on a scale of one to seven. The rating process Since 2017, we have asked the participants of the Expat Insider survey additional questions emphasized the respondents’ personal satisfaction with these aspects and considered about their city of residence. From the transportation options in their city to how easy it is for both emotional topics as well as more factual aspects with equal weight. The respondents’ them to find housing to the local career opportunities, the Expat City Ranking covers a wide ratings of the individual factors were then bundled in various combinations for a total of 13 range of topics related to expat life. subcategories, and their mean values were used to draw up four topical indices: Quality of The Expat City Ranking 2019 provides an in-depth analysis of 82 cities around the world. The Urban Living, Getting Settled, Urban Work Life, and Finance & Housing. These were further results focus on the quality of life, getting settled, work life, as well as finance and housing — averaged in order to rank cities worldwide. The survey also includes a Local Cost of Living providing an overview of the best and worst cities for expats worldwide. Index, which does, however, not factor into the overall ranking to avoid overrepresenting financial aspects. Short Methodology For a city to be featured in the Expat City Ranking 2019, a sample size of at least 50 survey This report is an addition to the annual country ranking featured in the Expat Insider survey participants per city was required. -

Social Policy
Perspective Paper Social Policy Harounan Kazianga First published 2011 Copenhagen Consensus Center Copenhagen, Denmark Rush Foundation, Lausanne, Switzerland © Copenhagen Consensus Center & Rush Foundation ISBN: 978-87-92795-21-2 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means electronic, mechanical, photo- copying, recording or otherwise, without the prior written permission of the Copenhagen Consensus Center and the Rush Foundation Perspective Paper Social Policy Harounan Kazianga1 1 Oklahoma State University RethinkHIV: The Project 2011 marks the 30-year anniversary since the Centers for Disease Control and Prevention introduced the world to the disease that became known as AIDS. Despite 30 years of increasing knowledge about transmission, prevention, and treatment, and current annual spending of $15 billion, every day around 7,000 people are infected with the HIV virus and two million die each year. The HIV/AIDS epidemic has had its most profound impact in sub- Saharan Africa, which accounts for 70 percent of new worldwide infections and 70 percent of HIV-related deaths, 1.8 million new infections in children each year, and has 14 million AIDS orphans. Humanitarian organizations warn that the fight against HIV/Aids has slowed, amid a funding shortfall and donor fatigue. Yet HIV is still the biggest killer of women of reproductive age in the world, and of men aged 15-59 in sub-Saharan Africa. Time is ripe for a reassessment of current policy and expenditure. The Rush Foundation has asked the Copenhagen Consensus Center to commission a group of leading health academics to analyze HIV policy choices and identify the most effective ways to tackle the pandemic across sub-Saharan Africa. -

Research Strengths of Greater Copenhagen with Investment Prospects Background Report November 2016
Research strengths of Greater Copenhagen with investment prospects Background Report November 2016 Quantum Technology Wind energy and energy storage Protein science and bioinformatics Humans and technology Greater Copenhagen Food and Nanoscience Metabolism and diabetes fermentation Social Big Data Bacteriology Cancer Acoustics and Bioenergy ultrasound Contents Preface .......................................................................................................................................... 3 Chapter 1 Research strengths with investment prospects ........................................................... 4 1.1 The competitive situation of Greater Copenhagen ............................................................ 4 1.2 Objective and approach ...................................................................................................... 4 1.3 Research strengths with investment prospects .................................................................. 7 1.4 Guidance to reading the factsheets .................................................................................. 10 Chapter 2 Methodological approach .......................................................................................... 11 2.1 Bibliometric analysis and interviews with university management...................................... 11 2.2 Special research investments and tradition for industry-collaboration ............................... 13 2.3 Prioritising the 12 selected strengths .................................................................................. -

Social Policy: Going Upstream
Assessment Paper Social Policy: Going upstream Anna Vassall, Michelle Remme and Charlotte Watts First published 2011 Copenhagen Consensus Center Copenhagen, Denmark Rush Foundation, Lausanne, Switzerland © Copenhagen Consensus Center & Rush Foundation ISBN: 978-87-92795-14-4 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means electronic, mechanical, photo- copying, recording or otherwise, without the prior written permission of the Copenhagen Consensus Center and the Rush Foundation Assessment Paper Social Policy Anna Vassall1, Michelle Remme2 and Charlotte Watts3 1 Lecturer, Economics of HIV, London School of Hygiene and Tropical Medicine 2 Health Finance Specialist, London School of Hygiene and Tropical Medicine 3 Professor, Department of Global Health and Development, Faculty of Public Health and Policy, London School of Hygiene and Tropical Medicine RethinkHIV: The Project 2011 marks the 30-year anniversary since the Centers for Disease Control and Prevention introduced the world to the disease that became known as AIDS. Despite 30 years of increasing knowledge about transmission, prevention, and treatment, and current annual spending of $15 billion, every day around 7,000 people are infected with the HIV virus and two million die each year. The HIV/AIDS epidemic has had its most profound impact in sub- Saharan Africa, which accounts for 70 percent of new worldwide infections and 70 percent of HIV-related deaths, 1.8 million new infections in children each year, and has 14 million AIDS orphans. Humanitarian organizations warn that the fight against HIV/Aids has slowed, amid a funding shortfall and donor fatigue. -

Medicinal Chemistry and Chemical Biology Chimia 2013, 67, Nr
medicinal chemistry and chemical biology Chimia 2013, 67, Nr. 7/8 519 doi:10.2533/chimia.2013.519 Chimia 67 (2013) 519–543 © Schweizerische Chemische Gesellschaft MedicinalChemistry Chemical Biology MC001 Medicinal ChemistryChemical Bioloy MC002 SOM230:Anew therapeuticmodality for Cushing’s disease Directed evolution of bicyclic peptides fortherapeutic application Ian Lewis, Janos Pless, Rainer Kneuer, Antonio P. Silva, Daniel Hoyer, Gisbert Lisa Pollaro, Alessandro Angelini, KhanMaola andChristian Heinis Weckbecker, ChristianBruns and Herbert A. Schmid EPFL, BCH5305, 1015 Lausanne,Switzerland GlobalDiscovery Chemistry Department ExploratoryMedicinal Chemistry Unit andAutoimmune, Oncologyand Neuroscience Research Departments, Bicyclic peptideswith highbinding affinity andtargetselectivitycan be NovartisInstitutesofBiomedicalResearch, CH-4002 Basel,Switzerland isolated from large combinatoriallibrariesbyphage display as shown in the figurebelow. In brief,peptide librariesdisplayed on phage arecyclizedina The somatostatin (SRIF,somatotropinrelease inhibiting factor) field has chemical reactionand subjected to affinity selections. The bicyclic peptides been asuccess story in termsofmedicinal chemistryand drug discovery combine keyqualities of antibodytherapeutics (high affinity andspecificity) offering avariety of therapeutic opportunities, e.g. acromegaly, gastrointes- andsomeadvantagesofsmall molecule drugs. tinal neuroendocrine tumors, whole body imaging and radiotherapy. Indeed, arational medicinal chemistry approach capitalising on structure