Marine Ecology Progress Series 382:211
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Breeding Biology of the Horned Puffin on St. Lawrence Island, Bering Sea, with Zoogeographical Notes on the North Pacific Puffins I
Pacific Science (1973), Vol. 27, No.2, p. 99-119 Printed in Great Britain Breeding Biology of the Horned Puffin on St. Lawrence Island, Bering Sea, with Zoogeographical Notes on the North Pacific Puffins I SPENCER G. SEALY' THE HORNED PUFFIN (Fratercula corniculata) is one of six species ofalcids which regularly nest on Sevuokuk Mountain, 3 km east of Gambell on St. Lawrence Island, Alaska (Fig. 1). During the summers of 1966 and 1967, I conducted on this island a study of the breeding ecology of three of these species, the Parakeet Auklet (Cyc/orrf?ynchuspsittacula), Crested Auklet (Aethia cristatella), and Least Auklet (A. pusilla) (see Sealy, 1968). During these summers some ob servations on the breeding biology of the Horn ed Puffin were obtained and are reported here. The only life history study ofthis species which spans the entire breeding season is that of Swartz (1966) in the Cape Thompson region, Alaska, some 560 km north of St. Lawrence Island (Fig. 2). Numerous studies of the biology of the con generic Common Puffin (Fratercula arctica) of the Atlantic and Arctic oceans are available (e.g., Lockley, 1953; Be1opol'skii, 1957; Uspen ski, 1958; Myrberget, 1959, 1961, 1962; Kartas chew, 1960; Nettleship, 1972; and others) and some of these will be utilized here for compara tive purposes. When available, comparative ob servations on the breeding biology of the other Pacific puffins, the Rhinoceros Auklet (Ceror hinca monocerata), which is actually a puffin (Storer, 1945), and the Tufted Puffin (Lunda cirrhata) will also be included. DISTRIBUTION The breeding distribution of the Horned Puffin has been mapped recently by Udvardy (1963: 105). -

PICES-2012 Program and Abstracts
PICES-2012 Program & Abstracts Image: “The Itsukushima Shrine from the sea”, November 25, 2010, watercolour, by Satoshi Arima, retiree of the National Research Institute of Fisheries and Environment of Inland Sea, Hiroshima, Japan. Permission to reproduce this artwork was kindly granted by Mr. Arima. Prepared and published by: North Pacific Marine Science Organization PICES Secretariat P.O. Box 6000 Sidney, BC PICES-2012 V8L 4B2 Canada Tel: 1-250-363-6366 Fax: 1-250-363-6827 Program and Abstracts E-mail: [email protected] Website: www.pices.int October 12-21, 2012 Hiroshima, Japan PICES-2012 Effects of natural and anthropogenic stressors in the North Pacific ecosystems: Scientific challenges and possible solutions North Pacific Marine Science Organization October 12-21, 2012 Hiroshima, Japan Table of Contents Notes for Guidance � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � �vi International Conference Center Floor Plan � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � vii Meeting Timetable � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � �viii Map of the International Conference Center Area � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � xii Keynote Lecture � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 1 Schedules and Abstracts S1: Science Board Symposium Effects of natural and anthropogenic stressors in -

SHOREBIRDS (Charadriiformes*) CARE MANUAL *Does Not Include Alcidae
SHOREBIRDS (Charadriiformes*) CARE MANUAL *Does not include Alcidae CREATED BY AZA CHARADRIIFORMES TAXON ADVISORY GROUP IN ASSOCIATION WITH AZA ANIMAL WELFARE COMMITTEE Shorebirds (Charadriiformes) Care Manual Shorebirds (Charadriiformes) Care Manual Published by the Association of Zoos and Aquariums in association with the AZA Animal Welfare Committee Formal Citation: AZA Charadriiformes Taxon Advisory Group. (2014). Shorebirds (Charadriiformes) Care Manual. Silver Spring, MD: Association of Zoos and Aquariums. Original Completion Date: October 2013 Authors and Significant Contributors: Aimee Greenebaum: AZA Charadriiformes TAG Vice Chair, Monterey Bay Aquarium, USA Alex Waier: Milwaukee County Zoo, USA Carol Hendrickson: Birmingham Zoo, USA Cindy Pinger: AZA Charadriiformes TAG Chair, Birmingham Zoo, USA CJ McCarty: Oregon Coast Aquarium, USA Heidi Cline: Alaska SeaLife Center, USA Jamie Ries: Central Park Zoo, USA Joe Barkowski: Sedgwick County Zoo, USA Kim Wanders: Monterey Bay Aquarium, USA Mary Carlson: Charadriiformes Program Advisor, Seattle Aquarium, USA Sara Perry: Seattle Aquarium, USA Sara Crook-Martin: Buttonwood Park Zoo, USA Shana R. Lavin, Ph.D.,Wildlife Nutrition Fellow University of Florida, Dept. of Animal Sciences , Walt Disney World Animal Programs Dr. Stephanie McCain: AZA Charadriiformes TAG Veterinarian Advisor, DVM, Birmingham Zoo, USA Phil King: Assiniboine Park Zoo, Canada Reviewers: Dr. Mike Murray (Monterey Bay Aquarium, USA) John C. Anderson (Seattle Aquarium volunteer) Kristina Neuman (Point Blue Conservation Science) Sarah Saunders (Conservation Biology Graduate Program,University of Minnesota) AZA Staff Editors: Maya Seaman, MS, Animal Care Manual Editing Consultant Candice Dorsey, PhD, Director of Animal Programs Debborah Luke, PhD, Vice President, Conservation & Science Cover Photo Credits: Jeff Pribble Disclaimer: This manual presents a compilation of knowledge provided by recognized animal experts based on the current science, practice, and technology of animal management. -

Submarine Foraging Behavior of Alcids in an Artificial Environment
Zoo Biology 6:373-378 (1987) Submarine Foraging Behavior of Alcids in an Artificial Environment David Cameron Duffy, Frank S. Todd, and W.R. Siegfried Percy FitzPatrick Institute of African Ornithology, University of Cape Town, Cape Town, South Africa (D.C.D., WR.S.); Hubbs Sea World Research Institute, San Diego, California (F.S. T.) We used an artificial environment at Sea World, Inc, San Diego, to study under water foraging behavior of a1cids. Larger birds dove longer and had greater wing beat frequencies. The pigeon guillemot Cepphus columba was the only species to use both feet and wings for propulsion; all others used just wings. Aggressive interactions underwater were common. Competition among alcids in the wild may occur primarily underwater, and artificial environments may be the best means to study such interactions. Key words: captivity, competition, diving, foraging INTRODUCTION The study of foraging by seabirds in their natural environments is extremely difficult as they can rarely be observed directly below the surface. Inferences con cerning foraging, based on surface observations, may lead to ambiguous results. For example, Cody [1973] concluded that differences in foraging range, rather than diet or foraging depth, facilitated partitioning of food resources between species in alcid communities off the Olympic Peninsula, Washington, and off Iceland. Bedard [1976] questioned Cody's findings but did not present alternative explanations for possible partitioning of resources. We suggest that, because alcids normally capture prey below the surface of the sea [Ashmole, 1971; Bradstreet and Brown, 1985], studies of the birds' submarine foraging behavior are needed. We report here an initial study of foraging by seven alcid species kept at Sea World, Inc, San Diego, in an artificial enclosure that attempts to simulate their natural environment. -

Adjustment of Provisioning According to Nestling Age and Mass
Flexible effort in breeding seabirds: adjustment of provisioning according to nestling age and mass Douglas F. Bertram, Clive V.J. Welham, and Ronald C. Ydenberg Abstract: We examined whether rhinoceros auklet (Cerorhinca monocarata) parents are able to adjust their provisioning effort in response to chick demand. A fostering experiment in which nestlings of different ages and masses were exchanged between burrows was employed to examine parental provisioning effort before and after the exchange. Daily mass increments of the nestlings were used to estimate the amount fed on the previous night, using a model based on data from captive chicks raised on a controlled diet. Our results demonstrate that rhinoceros auklet parents somehow assess and respond to the needs of their chicks by delivering more to older and larger fostered chicks and less to younger and smaller fostered chicks. Our results confirm and extend a growing body of information which shows that seabird parents can adjust provisioning effort when feeding young. We highlight how studies to date have differentially manipulated nestling age and hunger, thus complicating a comparative approach. Testing for interspecific differences in parental ability to res'pond to chick demands will require studies that employ comparable types of manipulations for a variety of seabird species. RCsumC : Nous avons tent6 d'Ctablir si, chez le Macareux rhinoceros (Cerorhinca monocarata), les parents sont capables d'ajuster leurs efforts d'approvisionnement de nourriture aux exigences de leurs oisillons. Nous avons procCdC B un Clevage expkrimental dans lequel des oisillons de masses et d'8ges divers ont CtC interchangks d'un terrier B l'autre et nous avons mesurC les efforts d'approvisionnement des parents avant et aprks 1'Cchange. -

Comparative Reproductive Ecology of the Auks (Family Alcidae) with Emphasis on the Marbled Murrelet
Chapter 3 Comparative Reproductive Ecology of the Auks (Family Alcidae) with Emphasis on the Marbled Murrelet Toni L. De Santo1, 2 S. Kim Nelson1 Abstract: Marbled Murrelets (Brachyramphus marmoratus) are breed on the Farallon Islands in the Pacific Ocean (Common comparable to most alcids with respect to many features of their Murre, Pigeon Guillemot, Cassin’s Auklet, Rhinoceros Auklet, reproductive ecology. Most of the 22 species of alcids are colonial and Tufted Puffin) are presented by Ainley (1990), Ainley in their nesting habits, most exhibit breeding site, nest site, and and others (1990a, b, c) and Boekelheide and others (1990). mate fidelity, over half lay one egg clutches, and all share duties Four inshore fish feeding alcids of the northern Pacific Ocean of incubation and chick rearing with their mates. Most alcids nest on rocky substrates, in earthen burrows, or in holes in sand, (Kittlitz’s Murrelet, Pigeon Guillemot, Spectacled Guillemot, around logs, or roots. Marbled Murrelets are unique in choice of and Marbled Murrelet) are reviewed by Ewins and others nesting habitat. In the northern part of their range, they nest on (1993) (also see Marshall 1988a for a review of the Marbled rocky substrate; elsewhere, they nest in the upper canopy of coastal Murrelet). The Ancient Murrelet, another inhabitant of the coniferous forest trees, sometimes in what appear to be loose northern Pacific Ocean, has been reviewed by Gaston (1992). aggregations. Marbled Murrelet young are semi-precocial as are Alcids that nest in small, loosely-aggregated colonies, as most alcids, yet they hatch from relatively large eggs (relative to isolated pairs, or in areas less accessible to researchers, have adult body size) which are nearly as large as those of the precocial not been well studied. -

The Curious Case of the Puffin Face by Morgan Barnes When Most Birds
The Curious Case of the Puffin Face By Morgan Barnes When most birds hear the word “winter”, they think of migrating several thousand miles to the warm tropics, or finding a nice, well-stocked bird feeder...but not the puffin. For these birds, winter means something a little different: part of their face is going to fall off! While that may sound concerning, it’s actually a normal part of the puffin’s life history. Before we delve into how and why these birds lose part of their face, let’s learn a bit An adult Horned Puffin in breeding plumage; inset: more about puffins. adult Horned Puffin after shedding bill, and losing breeding plumage. There are four species of puffin: the Photo credit: Morgan Barnes Atlantic Puffin, Horned Puffin, Tufted Puffin, and the Rhinoceros Auklet. These birds live a double life, spending their summers nesting on rocky cliffs and islands, and spending their winters foraging out to sea. Puffins are round, stocky birds, often described as “flying footballs”. During spring and summer, puffins display their characteristic brightly colored bills, with oranges, yellows and reds. Their feathers, called plumage, are a handsome solid black, with varying amounts of white plumage that differs between species. Unlike many birds, both male and female puffins show this combination of brightly colored bill and contrasting body plumage. When puffins return from the open ocean in spring, their colorful bills can only mean one thing: it’s time to settle down and raise a chick. After a swim together, a pair of puffins will touch beaks. -

Breeding Biology of the Rhinoceros Auklet in Washington’
TheCondor88:143-155 0 The Cooper Ornithological Society 1986 BREEDING BIOLOGY OF THE RHINOCEROS AUKLET IN WASHINGTON’ ULRICH W. WILSON U.S. Fish and Wildlife Service,Nisqually National Wildrif Refuge, 100 Brown Farm Rd., Olympia, WA 98506 DAVID A. MANUWAL Wildrife ScienceGroup, Collegeof Forest Resources,University of Washington,Seattle, WA 98195 Abstract. During 1914 through 1983, we investigated the breeding biology of the Rhinoceros Auklet (Cerorhincamonocerata) at three main colony siteson the coastof Washington:Destruction Island (offshore) and Protection and Smith islands (inland islands of the Strait of Juan de Fuca). Averageburrow densitieswere higher offshore,where the aukletsnested on shrub-coveredslopes; inland aukletsnested on grassyslopes and level areas. Egg-layingpatterns varied among years and populations,although initiation dates on all islandswere similar. The incubation periods averaged 45 days and ranged from 39 to 52 days. Chicks were brooded, on average,for 3.9 days (rangezero to 9 days). On Protection Island, early-hatched young grew more rapidly than chicks hatched at a later date. Chicks on offshore islands were fed a variety of fish, whereas those on inland islands were fed primarily two species.The inland chicks were fed heavier fish loads, reachedheavier peak body weights, and were heavier when they fledgedthan were offshore chicks. Breeding successwas higher on the inland colony sites. Key words: RhinocerosAuklet; Cerorhinca monocerata; Washington(state); breeding biology; pujins. INTRODUCTION DESCRIPTION OF STUDY SITES Among the Pacific alcids, the puffins show Destruction Island (47”40’N, 124”24’W) is lo- strong structural (Storer 1945) and ecological cated 4.8 km west of the Olympic Peninsula similarities that provide an opportunity to ex- and 29 km south-southeastof La Push, Wash- amine the species’ responsesto their environ- ington (Fig. -

Threats to Seabirds: a Global Assessment 2 3 4 Authors: Maria P
1 Threats to seabirds: a global assessment 2 3 4 Authors: Maria P. Dias1*, Rob Martin1, Elizabeth J. Pearmain1, Ian J. Burfield1, Cleo Small2, Richard A. 5 Phillips3, Oliver Yates4, Ben Lascelles1, Pablo Garcia Borboroglu5, John P. Croxall1 6 7 8 Affiliations: 9 1 - BirdLife International. The David Attenborough Building, Pembroke Street Cambridge CB2 3QZ UK 10 2 - BirdLife International Marine Programme, RSPB, The Lodge, Sandy, SG19 2DL 11 3 – British Antarctic Survey. Natural Environment Research Council, High Cross, Madingley Road, 12 Cambridge CB3 0ET, UK 13 4 – Centre for the Environment, Fishery and Aquaculture Science, Pakefield Road, Lowestoft, NR33, UK 14 5 - Global Penguin Society, University of Washington and CONICET Argentina. Puerto Madryn U9120, 15 Chubut, Argentina 16 * Corresponding author: Maria Dias, [email protected]. BirdLife International. The David 17 Attenborough Building, Pembroke Street Cambridge CB2 3QZ UK. Phone: +44 (0)1223 747540 18 19 20 Acknowledgements 21 We are very grateful to Bartek Arendarczyk, Sophie Bennett, Ricky Hibble, Eleanor Miller and Amy 22 Palmer-Newton for assisting with the bibliographic review. We thank Rachael Alderman, Pep Arcos, 23 Jonathon Barrington, Igor Debski, Peter Hodum, Gustavo Jimenez, Jeff Mangel, Ken Morgan, Paul Sagar, 24 Peter Ryan, and other members of the ACAP PaCSWG, and the members of IUCN SSC Penguin Specialist 25 Group (Alejandro Simeone, Andre Chiaradia, Barbara Wienecke, Charles-André Bost, Lauren Waller, Phil 26 Trathan, Philip Seddon, Susie Ellis, Tom Schneider and Dee Boersma) for reviewing threats to selected 27 species. We thank also Andy Symes, Rocio Moreno, Stuart Butchart, Paul Donald, Rory Crawford, 28 Tammy Davies, Ana Carneiro and Tris Allinson for fruitful discussions and helpful comments on earlier 29 versions of the manuscript. -

Marine Atlas of Pacific Canada 142°W 140°W 138°W 136°W 134°W 132°W 130°W 128°W 126°W 124°W 122°W
data sources • Environment Canada (Canadian Wildlife Service) – British Columbia Seabird Colony Inventory Marine Birds – Rhinoceros Auklet Colonies • Parks Canada – Nesting Seabird Colonies data resolution • 1:250,000 (some points have been adjusted to match the 1:20,000 TRIM coastline) description The Rhinoceros Auklet (Cerorhinca monocerata) is actually a puffin. Closely related to the Tufted Puffin, the Rhinoceros Auklet is a large data collected alcid with a wedge-shaped head. It is drab-grey, darker above than below, with a thick yellow bill that is adorned with a white horn during the • 1977-1993 (years breeding was confirmed) breeding season. Rhinoceros Auklets are found both in coastal habitats and far from land. They often feed close to shore, especially where tidal currents near islands create upwellings and concentrations of food. Rhinoceros Auklets nest in burrows in the ground on both forested and date compiled non-forested islands. • 2008 This atlas page depicts active and historic Rhinoceros Auklet colonies in terms of their relative importance, a value that corresponds to the number of “nest pairs” estimated at any one site during the most recent survey year. This value was compared to the total BC population, which reviewers was determined by summing the estimated number of individuals at all colonies during the most recent survey year. Using these values, relative • Harry Carter, Consultant importance was determined as follows: • Peter Davidson, Bird Studies Canada • Representatives from Environment Canada, Canadian Wildlife Service • High: colony has >5% of the species’ total BC population. • Moderate: colony has 1-5% of the species’ total BC population. reviewer comments • Low: colony has <1% of the species’ total BC population. -

University Microfilms International 300 N
The Breeding Biology Of The Puffins: Tufted Puffin (Lunda Cirrhata), Horned Puffin (Fratercula Corniculata), Common Puffin (F. Arctica), And Rhinoceros Auklet (Cerorhinca Monocerata) Item Type Thesis Authors Wehle, Duff Henry Strong Download date 09/10/2021 09:57:35 Link to Item http://hdl.handle.net/11122/9312 INFORMATION TO USERS This was produced from a copy of a document sent to us for microfilming. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the material subm itted. The following explanation of techniques is provided to help you understand markings or notations which may appear on this reproduction. 1. Trie sign or “ target” fo r pages apparently lacking from the docum ent photographed is “Missing Page(s)”. If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting through an image and duplicating adjacent pages to assure you of complete continuity. 2. When an image on the film is obliterated with a round black mark it is an indication that the film inspector noticed either blurred copy because of movement during exposure, or duplicate copy. Unless we meant to delete copyrighted materials that should not have been filmed, you will find a good image of the page in the adjacent frame. If copyrighted materials were deleted you will find a target note listing the pages in the adjacent frame. 3. When a map, drawing or chart, etc., is part of the material being photo graphed the photographer has followed a definite method in “sectioning” the material. -
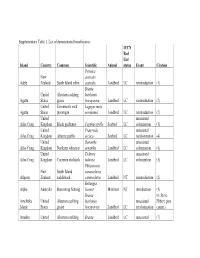
Supplementary Table 1. List of Demonstrated Beneficiaries
Supplementary Table 1. List of demonstrated beneficiaries. IUCN Red List Island Country Common Scientific Animal status Event Citation Petroica New australis Adele Zealand South Island robin australis Landbird LC reintroduction (1) Branta United Aleutian cackling hutchinsii Agattu States goose leucopareia Landbird LC reintroduction (2) United Evermann's rock Lagopus muta Agattu States ptarmigan evermanni Landbird LC reintroduction (2) United unassisted Ailsa Craig Kingdom Black guillemot Cepphus grylle Seabird LC colonization (3) United Fratercula unassisted Ailsa Craig Kingdom Atlantic puffin arctica Seabird LC recolonization (4) United Oenanthe unassisted Ailsa Craig Kingdom Northern wheatear oenanthe Landbird LC colonization (4) United Tadorna unassisted Ailsa Craig Kingdom Common shelduck tadorna Landbird LC colonization (3) Philesturnus New South Island carunculatus Allports Zealand saddleback carunculatus Landbird NT reintroduction (2) Bettongia Alpha Australia Burrowing bettong lesueur Mammal NT introduction (5) Branta (6; Steve Amchitka United Aleutian cackling hutchinsii unassisted Ebbert, pers. Island States goose leucopareia Landbird LC recolonization comm.) Amukta United Aleutian cackling Branta Landbird LC unassisted (7) IUCN Red List Island Country Common Scientific Animal status Event Citation States goose hutchinsii recolonization leucopareia Sally Amy Poncet, Island/Outer United Cinclodes unassisted unpublished Knob Kingdom Tussacbird antarcticus Landbird LC recolonization data Sally Amy Poncet, Island/Outer United unpublished