Chrysin Pretreatment Improves Angiotensin System, Cgmp Concentration in L-NAME Induced Hypertensive Rats
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Telephone Numbers
DISTRICT DISASTER MANAGEMENT AUTHORITY THANJAVUR IMPORTANT TELEPHONE NUMBERS DISTRICT EMERGENCY OPERATION CENTRE THANJAVUR DISTRICT YEAR-2018 2 INDEX S. No. Department Page No. 1 State Disaster Management Department, Chennai 1 2. Emergency Toll free Telephone Numbers 1 3. Indian Meteorological Research Centre 2 4. National Disaster Rescue Team, Arakonam 2 5. Aavin 2 6. Telephone Operator, District Collectorate 2 7. Office,ThanjavurRevenue Department 3 8. PWD ( Buildings and Maintenance) 5 9. Cooperative Department 5 10. Treasury Department 7 11. Police Department 10 12. Fire & Rescue Department 13 13. District Rural Development 14 14. Panchayat 17 15. Town Panchayat 18 16. Public Works Department 19 17. Highways Department 25 18. Agriculture Department 26 19. Animal Husbandry Department 28 20. Tamilnadu Civil Supplies Corporation 29 21. Education Department 29 22. Health and Medical Department 31 23. TNSTC 33 24. TNEB 34 25. Fisheries 35 26. Forest Department 38 27. TWAD 38 28. Horticulture 39 29. Statisticts 40 30. NGO’s 40 31. First Responders for Vulnerable Areas 44 1 Telephone Number Officer’s Details Office Telephone & Mobile District Disaster Management Agency - Thanjavur Flood Control Room 1077 04362- 230121 State Disaster Management Agency – Chennai - 5 Additional Cheif Secretary & Commissioner 044-28523299 9445000444 of Revenue Administration, Chennai -5 044-28414513, Disaster Management, Chennai 044-1070 Control Room 044-28414512 Emergency Toll Free Numbers Disaster Rescue, 1077 District Collector Office, Thanjavur Child Line 1098 Police 100 Fire & Rescue Department 101 Medical Helpline 104 Ambulance 108 Women’s Helpline 1091 National Highways Emergency Help 1033 Old Age People Helpline 1253 Coastal Security 1718 Blood Bank 1910 Eye Donation 1919 Railway Helpline 1512 AIDS Helpline 1097 2 Meteorological Research Centre S. -
List of Polling Stations for 176 Pattukkottai Assembly Segment Within the 30 Thanjavur Parliamentary Constituency
List of Polling Stations for 176 Pattukkottai Assembly Segment within the 30 Thanjavur Parliamentary Constituency Sl.No Polling Location and name of building in Polling Areas Whether for All station No. which Polling Station located Voters or Men only or Women only 12 3 4 5 1 1 Panchayat Union Elementary 1.Nemmeli ( R.V) And (P) South Street wd 1 , 2.Nemmeli ( R.V) And (P) Middle All Voters School West Facing Terraced Street Wd 2 , 3.Nemmeli ( R.V) And (P) Northstreet Wd 3 , 4.Nemmeli ( R.V) And Building, ,Nemmeli 614015 (P) Adi Dravidar street Wd 4 2 2 Panchayat Union Middle School 1.Keelakurichi West (R.V) And (P) Subramaniyarkovil street, Bank street, All Voters North East Facing West Terraced Adidravidar Colony wd 1 , 2.Keelakurichi West (R.V) and (P) Sivankovil Steet Wd 1 Building, ,Keelakkurichi 614015 , 3.Keelakurichi West (R.V) and (P) Middlestreet Wd 1 , 4.Keelakurichi West (R.V) and (P) South Street Wd 2 , 5.Keelakurichi West (R.V) and (P) North Street Wd 2 , 6.Keelakurichi West (R.V) and (P) Thenmelavadkku theru Wd 2 , 7.Keelakurichi West (R.V) and (P) New South Steet Wd 2 3 3 Panchayat Union Elementay 1.Keelakurichi East (R.V) AND (P) West Street, Sivankovil Street, Middle Street, All Voters School South Facing North North Street , 2.Keelakurichi East (R.V) AND (P) Adidravidar Street, Annanagar Main Terrraced Building, road Wd 3 ,Keelakkurichi 614015 4 4 Panchayat Union Elementary 1.Keelakurichi West (R.V) AND (P) Mandalakkottai ward 1 All Voters School South Building East Facing, ,Mandalakkottai 614015 5 5 Panchayat Union -

Khadi Institution Profile Khadi and Village Industries
KHADI AND VILLAGE INDUSTRIES COMISSION KHADI INSTITUTION PROFILE Office Name : SO CHENNAI TAMIL NADU Institution Code : 4529 Institution Name : THANJAVUR WEST SARVODAYA SANGH Address: : 28-GIRI ROAD, SRINIVASAPURAM Post : SRINIVASAPURAM City/Village : THANJAVUR Pincode : 613009 State : TAMIL NADU District : THANJAVUR Aided by : KVIC District : B Contact Person Name Email ID Mobile No. Chairman : R GANESAN 9443722414 Secretary : RS SIVAKUMAR 9865561337 Nodal Officer : Registration Detail Registration Date Registration No. Registration Type 01-01-1111 15/1974 SOC Khadi Certificate No. TND/3063 Date : 31-MAR_2021 Khadi Mark No. KVIC/CKMC/TN029 Khadi Mark Dt. 01-Oct-2019 Sales Outlet Details Type Name Address City Pincode Sales Outlet KHADI GRAMODYOG 87/88 GANDHI MANNARGUDI 614001 BHAVAN ROAD, MANNARGUDI Sales Outlet KHADI VASTRALAYA PAZHAMANNERI, TIRUKKATTUPPALL 613104 I Sales Outlet KHADI GRAMODYOG 41,BIG STREET PATTUKKOTTAI 614701 BHAVAN Sales Outlet GRAMODYOG SALES DEPOT 41 NATESAN ST, MANNARGUDI 614001 SAKTHI COMPELXS Sales Outlet KHADI GRAMODYOG VALLAM IROAD, R S THANJAVUR 613005 BHAVAN G COLLAGE Sales Outlet KHADI GRAMODYOG SOUTH RAMPART THANJAVUR 613001 BHAVAN,SILK PALACE VANIGAMAIYAM Sales Outlet KHADI GRAMODYOG 1306 SOUTH MAIN THANJAVUR 613009 BHAVAN STREET, Sales Outlet GRAMODOYA SALES DEPO, K.G.COMPELX. VEDARANYAM 615703 NORTH STREET Sales Outlet KHADI VASTRALAYA SETHU RASTHA VEDARANYAM 613009 Sales Outlet KHADI VASTRALAYA MMA COMPELX, ARANTHANGI 614616 THALUKKA OFFICE ROAD Sales Outlet KHADI VASTRALAYA K.V. COMPLEX ORATHANADU 614625 BUSTANT OPP. , ORATHANADU 26 September 2021 Page 1 of 3 Sales Outlet KHADI GRAMODYOG 106 SOUTH STREET THANJAVUR 613204 BHAVAN Sales Outlet BRASS LAMP SHOW ROOM RSG COLLOGE PO THANJAVUR 613005 Infrastructure Details Infrastructure Type Description in No. -

Trichy FOC Centres Phone Numbers Land Line Mobile 9445853
Name of the Region : Trichy Fuse Off Call Centres Name of the Circle : Trichy Phone Numbers FOC Centres Land Line Mobile For BSNL Users:1912 9445853479 - TRICHY For others:04311912 9445853480 Name of the Circle : Karur Phone Numbers FOC Centres Land Line Mobile KARUR 1912 94445854093 Name of the Circle : Pudukkottai Phone Numbers FOC Centres Land Line Mobile Pudukkottai 04322-221523 ----- Landline Numbers Name of the Circle : TRICHY Elecy. Distn. Circle/Metro Section Sub-Division Division Section Name Phone No Sub Division Phone No Division Phone No Name Name Thennur 0431-2794237 Thillainagar 0431-2791467 0431 - Woraiyur 0431 -2794237 THENNUR 2794237 Srinivasanagar 0431 -2794237 Con-II/Rockfort 0431-2793220 Con-I/Urban/Trichy 0431-2793220 Rockfort 0431-2793220 0431- Cinthamani 0431 -2793220 ROCKFORT 0431 - 2793220 Maingauardgate 0431 -2793220 URBAN 2793131 110KV/K.Pettai 0431 -2706443 Palakkarai 0431-2793220 Gandhimarket 0431-2793220 Senthaneerpuram 0431 -2793220 0431 - PALAKKARAI Junction 0431 -2414749 2793220 Ponnagar 0431-2481858 Mahalakshminagar 0431 -2202525 Cantonment 0431-2460148 Mannarpuram 0431-2420145 Subramaniapuram 0431 -2420145 Up graded Code No: Sembattu 0431 -2341924 section 0431 Crawford 0431 -2471880 KK Nagar 0431 -2341032 Rural/ Trichy 0431-2422301 EAST 0431 Manikandam 0431-2680300 /TRICHY 242223 Tiruparaithurai 0431-2614322 RURAL / 0431- TRICHY 2422301 Somarasampettai 0431-2607271 110 KV SS/ Ammapettai 0431-2680300 110 KV SS/Alundur 0431-2680514 Tiruverumbur 0431-2512773 THIRUVERUMB 0431- Navalpattu 0431-2512224 UR -

1 1. Location of Offices of Transport Department
1. Location of Offices of Transport Department 1 2. ORGANISATION CHART OF THE TRANSPORT DEPARTMENT (HEAD QUARTERS SET UP) TRANSPORT COMMISSIONER / STATE TRANSPORT AUTHORITY Addl.Trpt.Commr., J.T.C. (Admin) J.T.C. (R) J.T.C.(R.S) D.T.C- Admin. D.T.C- I D.T.C- II C.A.O L.O A.S- I A.S- II A.S- III A.D.S A.O A.D.(IAW) S/S.T.A.T D.R.S.T.A.T 2 ORGANISATION CHART OF THE TRANSPORT DEPARTMENT Transport Commissioner JTC (North Zone) JTC (South Zone) RTOs- 10 AAO-1 RTOs- 12 AAO-1 UO - 2 UO - 2 Check Posts -4 Check Posts -0 Villupuram Zone Vellore Zone RTOs- 8 AAO-1 RTOs- 6 AAO-1 UO - 6 UO - 4 Check Posts -1 Check Posts -6 Salem Zone Erode Zone RTOs- 8 AAO-1 RTOs- 8 AAO-1 UO - 4 UO - 5 Check Posts -1 Check Post -1 JTC, Coimbatore zone Thanjavur Zone RTOs- 11 AAO-1 RTOs- 7 AAO-1 UO - 6 UO - 7 Check Posts -6 Check Post -0 Trichy Zone JTC, Madurai zone RTOs- 7 AAO-1 RTOs- 7 AAO-1 UO -8 UO - 9 Check Posts -0 Check Post -1 Virudhunagar Zone Tirunelveli zone RTOs- 6 AAO-1 RTOs-8 AAO-1 UO-3 UO -2 Check Post -2 JTC (Enforcement) -2 STA- Please see Previous page 3 3. OFFICES FUNCTIONING UNDER THE CONTROL OF TRANSPORT DEPARTMENT Number of Offices: 180 Zonal Offices :12, Enfo Offices:2, RTO Offices: 87, Unit Offices:58, Check Posts :21 Sl. -
List of Blocks of Tamil Nadu District Code District Name Block Code
List of Blocks of Tamil Nadu District Code District Name Block Code Block Name 1 Kanchipuram 1 Kanchipuram 2 Walajabad 3 Uthiramerur 4 Sriperumbudur 5 Kundrathur 6 Thiruporur 7 Kattankolathur 8 Thirukalukundram 9 Thomas Malai 10 Acharapakkam 11 Madurantakam 12 Lathur 13 Chithamur 2 Tiruvallur 1 Villivakkam 2 Puzhal 3 Minjur 4 Sholavaram 5 Gummidipoondi 6 Tiruvalangadu 7 Tiruttani 8 Pallipet 9 R.K.Pet 10 Tiruvallur 11 Poondi 12 Kadambathur 13 Ellapuram 14 Poonamallee 3 Cuddalore 1 Cuddalore 2 Annagramam 3 Panruti 4 Kurinjipadi 5 Kattumannar Koil 6 Kumaratchi 7 Keerapalayam 8 Melbhuvanagiri 9 Parangipettai 10 Vridhachalam 11 Kammapuram 12 Nallur 13 Mangalur 4 Villupuram 1 Tirukoilur 2 Mugaiyur 3 T.V. Nallur 4 Tirunavalur 5 Ulundurpet 6 Kanai 7 Koliyanur 8 Kandamangalam 9 Vikkiravandi 10 Olakkur 11 Mailam 12 Merkanam Page 1 of 8 List of Blocks of Tamil Nadu District Code District Name Block Code Block Name 13 Vanur 14 Gingee 15 Vallam 16 Melmalayanur 17 Kallakurichi 18 Chinnasalem 19 Rishivandiyam 20 Sankarapuram 21 Thiyagadurgam 22 Kalrayan Hills 5 Vellore 1 Vellore 2 Kaniyambadi 3 Anaicut 4 Madhanur 5 Katpadi 6 K.V. Kuppam 7 Gudiyatham 8 Pernambet 9 Walajah 10 Sholinghur 11 Arakonam 12 Nemili 13 Kaveripakkam 14 Arcot 15 Thimiri 16 Thirupathur 17 Jolarpet 18 Kandhili 19 Natrampalli 20 Alangayam 6 Tiruvannamalai 1 Tiruvannamalai 2 Kilpennathur 3 Thurinjapuram 4 Polur 5 Kalasapakkam 6 Chetpet 7 Chengam 8 Pudupalayam 9 Thandrampet 10 Jawadumalai 11 Cheyyar 12 Anakkavoor 13 Vembakkam 14 Vandavasi 15 Thellar 16 Peranamallur 17 Arni 18 West Arni 7 Salem 1 Salem 2 Veerapandy 3 Panamarathupatti 4 Ayothiyapattinam Page 2 of 8 List of Blocks of Tamil Nadu District Code District Name Block Code Block Name 5 Valapady 6 Yercaud 7 P.N.Palayam 8 Attur 9 Gangavalli 10 Thalaivasal 11 Kolathur 12 Nangavalli 13 Mecheri 14 Omalur 15 Tharamangalam 16 Kadayampatti 17 Sankari 18 Idappady 19 Konganapuram 20 Mac. -

Trichy, Location Tamilnadu
TIRUCHIRAPPALLI COMMISSIONERATE The jurisdiction of Tinrchirapalli Commissionerate covers the areas covering the entire Districts of Tiruchirappalli, Perambalur, Ariyalur, Karur, Pudukottai, Thanjavur, Thiruvarur, Nagapattinarn, Cuddalore and D. Gudalur village of Palayam Firka of Vedasandur Taluk of Dindigul District in the State of Tamil Nadu. Location I NO: 1, WILLIAMS ROAD, CANTONMENT, TRICI{Y- 620001. Divisions under the jurisdiction of Tiruchirapalli Commissionerate Sl.No. Divisions 1. Tiruchirapalli I Division 2. Tiruchirapalli II Division 3. Thanjavur Division 4. Karur Division 5. Cuddalore Division Pagc 62 of 83 1. Tiruchirappalli - I Division of Tiruchirapalli Commissionerate. 1st Floor, 'B'- Wing, 1, Williams Road, Cantonment, Trichy, Location Tamilnadu. PIN- 620 OOL. Areas covering Trichy District faltng on the southern side of Jurisdiction Kollidam river, Mathur, Mandaiyoor, Kalamavoor, Thondaimanallur and Nirpalani villages of Kolathur Taluk and Viralimalai Taluk of Pudukottai District. The Division has seven Ranges with jurisdiction as follows: Name of the Location Jurisdiction Range Areas covering Wards No. 7 to 25 of City - 1 Range Tiruchirappalli Municipal Corporation Areas covering Wards No.27 to 30, 41, 42, City - 2 Range 44, 46 to 52 of Tiruchirappalli Municipal l"t Floor, B- Wing, 1, Corporation Williams Road, Areas covering Wards No. 26, 31 to 37 43, Cantonment, Trichy, PIN , 54 to 60 of Tiruchirappalli Municipal 620 00L. Corporation; and Sempattu village of Trichy Taluk, Gundur, Sooriyur villages of City - 3 Range Tiruverumbur Taluk of Trichy District, Mathur, Mandaiyur, Kalamavoor, Thondamanallur, Nirpalani Village of Kulathur Taluk of Pudukottai District. Areas covering Wards No. 63 to 65 of Civil Maintenance Tiruverumbur Tiruchirappalli Municipal Corporation and Building, Kailasapuram, Range Navalpattu and Vengur villages of Trichy, PIN 620 OI4. -

List of Coconut Producers Societies in Tamil Nadu
List of Coconut Producers Societies in Tamil Nadu No CDB Reg Address Farmers Area Production CDB/TN/CBE/2 Kappalankarai Coconut Farmers Welfare Society, Kappalankarai (PO), 1 28 73.69 1103315 012-13/001 Negamam (Via), Kinathukadavu Taluk, Coimbatore, Tamil Nadu-642120 CDB/TN/CBE/2 Pasumai Coconut Producers Society, Perumpathi, Zaminkaliyapuram 2 108 387.49 105022 012-13/002 (PO), Pollachi Taluk, Coimbatore, Tamil Nadu-642110 Kottur Malayandipattinam Coconut Producers Welfare Society, 20/178- CDB/TN/CBE/2 3 Main Road, Kottur Malayandipattinam, Pollachi Taluk, Coimbatore, Tamil 30 108.77 1554000 013-14/003 Nadu-642114 CDB/TN/CBE/2 Devampadi Coconut Producers Society, 4/27-Devampadi Valasu, 4 26 53.24 889200 013-14/004 Pollachi, Coimbatore, Tamil Nadu-642005 Soolakkal Mettupalayam Coconut Producers Society, 2/105-Main Road, CDB/TN/CBE/2 5 Soolakkal Mettupalayam (PO), Kovilpalayam (Via), Pollachi Taluk, 34 117.27 2119954 013-14/005 Coimbatore, Tamil Nadu-642110 CDB/TN/CBE/2 Senbagam Coconut Producers Society, Thondamuthur (PO), , 6 25 60.52 1021800 013-14/006 Coimbatore, Tamil Nadu-642123 CDB/TN/CBE/2 Thamarai Coconut Farmers Welfare Society, Karuppampalayam (PO), , 7 25 39 856800 013-14/007 Coimbatore, Tamil Nadu-642004 CDB/TN/CBE/2 Karpagatharu Coconut Producers Society, Samathur (PO), Pollachi Taluk, 8 29 72.06 1244970 013-14/008 Coimbatore, Tamil Nadu-642123 CDB/TN/CBE/2 Rangasamuthiram Coconut Producers Society, Suleswaranpatti (PO), 9 40 89.04 1423877 013-14/009 Pollachi Taluk, Coimbatore, Tamil Nadu-642006 CDB/TN/CBE/2 Seelakkampatti Coconut Producers Society, Seelakkampatti (PO), 10 25 66.12 1175600 013-14/010 Pollachi Taluk, Coimbatore, Tamil Nadu-642205 CDB/TN/CBE/2 Ponnachiyur Coconut Producers Welfare Society, Samathur (PO), 11 29 101.62 1986000 013-14/011 Pollachi Taluk, Coimbatore, Tamil Nadu-642123 CDB/TN/CBE/2 S. -
Deo &Ceo Address List
EDU REVENUE SCHOOL DIST DISTRICT CODE SCHOOL NAME USERNAME DEO NAME AND ADDRESS KANYAKUMARI PUBLIC SCHOOL, KARUNIAPURAM, DISTRICT EDUCATIONAL OFFICER 01 01 50189 KANYAKUMARI C50189 THUCKALAY - 629 175 04651-250968 ST.JOSEPH CALASANZ SCHOOL SAHAYAMATHA DISTRICT EDUCATIONAL OFFICER 01 01 46271 STREET AGASTEESWARAM C46271 THUCKALAY - 629 175 04651-250968 GNANA VIDYA MANDIR MADUSOOTHANAPURAM VILLAGE KEEZHAKATTUVILAI, DISTRICT EDUCATIONAL OFFICER 01 01 46345 THENGAMPUTHUR C46345 THUCKALAY - 629 175 04651-250968 ST. JOSEPH'S SCHOOL ATTINKARAI, MANAVALAKURICHY DISTRICT EDUCATIONAL OFFICER 01 01 46362 KALKULAM C46362 THUCKALAY - 629 175 04651-250968 SMR NATIONAL SCHOOL LOUTS PARK, POST CHERUPPALOOR KULASEKHARAM, TEH DISTRICT EDUCATIONAL OFFICER 01 01 46383 KALKULAM C46383 THUCKALAY - 629 175 04651-250968 EXCEL CENTRAL SCHOOL, THIRUVATTAR, DISTRICT EDUCATIONAL OFFICER 01 01 50202 KANYAKUMARI C50202 THUCKALAY - 629 175 04651-250968 COMORIN INTERNATIONAL SCHOOL, ARALVAIMOZHI, DISTRICT EDUCATIONAL OFFICER 01 01 50211 KANYAKUMARI C50211 THUCKALAY - 629 175 04651-250968 SBJ VIDYA BHAVAN, PEACE GARDEN, KULASEKHARAM, DISTRICT EDUCATIONAL OFFICER 01 01 50216 KANYAKUMARI C50216 THUCKALAY - 629 175 04651-250968 SACRED HEART INTERNATIONAL SCHOOL, MARTHANDAM, DISTRICT EDUCATIONAL OFFICER 01 01 50221 KANYAKUMARI C50221 THUCKALAY - 629 175 04651-250968 CORPUS CHRISTI SCHOOL, PERUVILLAI P.O, DISTRICT EDUCATIONAL OFFICER 01 01 50222 KANYAKUMARI C50222 THUCKALAY - 629 175 04651-250968 EXCEL CENTRAL SCHOOL, A AWAI FARM LANE, THIRUVATTAR, DISTRICT EDUCATIONAL OFFICER -

Thanjavur District Industrial Profile 2020-21
Ministry of Micro, Small and Medium Enterprises Government of India Thanjavur District Industrial Profile 2020-21 Prepared by MSME Development Institute - Chennai Office of the Development Commissioner Ministry of Micro Small and Medium Enterprises Government of India INDEX CHAPTER CONTENT PAGE NO. 1 DISTRICT AT A GLANCE 4 2 INTRODUCTION 10 3 AVAILABLITY OF RESOURCES 16 4 INFRASTRUCTURE FACILITY EXISTING IN 23 THANJAVUR 5 INDUSTRIAL SCENARIO AND MSMEs AT 25 THANJAVUR 6 MICRO SMALL ENTERPRISES- CLUSTER 33 DEVELOPMENT PROGRAMME 7 SWOT ANALYISIS FOR ENTERPRISES 36 DEVELOPMENT 8 INSTITUTIONAL SUPPORT FOR MSMEs 37 9 STEPS TO SET UP ENTERPRISES 44 10 IMPORTANT SCHEMES AND ITS PERFORMANCE 59 11 ADDITIONAL INFORMATION ANNEXURE- ADDRESSESS OF CENTRAL AND STATE GOVT 67 I AUTHORITIES ANNEXURE- IMPORTANT CONTACTS IN THANJAVUR 71 II 2 DISTRICT MAP - THANJAVUR DISTRICT 3 CHAPTER-I THANJAVUR DISTRICT AT A GLANCE 1. PHYSICAL & ADMINISTRATIVE FEATURES Total Geographical Area (Sq.km) 3397 Division Taluks Thanjavur 1 Thanjavur 2 Orathanadu 3 Thiruvaiyaru 4 Budalur Kumbakonam 5 Kumbakonam 6 Papanasam 7 Thiruvidaimaruthur Pattukottai 8 Pattukottai 9 Peravurani Firkas 50 Revenue Villages 906 2. SOIL & CLIMATE Agro-climatic Zone Humid Tropical climate, Zone XI Climate Hot & Humid Soil Type Mainly alluvial 3. LAND UTILISATION [Ha] - (DSH - 2013-14) Total Area Reported 339657 Forest Land 3390 Area Not Available for Cultivation 83879 Permanent Pasture and Grazing Land 1218 Land under Miscellaneous Tree Crops 5337 Cultivable Wasteland 12097 Current Fallow 17943 Other Fallow 28458 Net Sown Area 187335 Total or Gross Cropped Area 267747 Area Cultivated More than Once 80412 Cropping Inensity [GCA/NSA] 143 4 4. RAINFALL & GROUND WATER (DSH - 2013-14) Rainfall [in Normal Actual 2011-12 2012-13 2013-14 mm] 1013 874 757 756 Variation from -13.7% -25.3% -25.4% Normal Availability Net Net Balance of Ground annual annual Water [Ham] recharge draft 73605 52788 20817 5. -

Farm Machine Dealers - Tamilnadu (------All Districts ------)
FARM MACHINE DEALERS - TAMILNADU (------ ALL DISTRICTS ------) Sl.No Name and address Products Contacts 1 Sri Venkateswara Agencies Diesel engines, Paddy Ph: 04112222172 Address: 79-A,Nellukkara Transpalnter, Power tiller, Rice Mob: 9443349922 Street,Kanchipuram-681502 transplanter, Self propelled Web: power weeder, Self propelled [email protected] reaper,Self-propelled paddy m transplanter, Self propelled rice transplanter, Trolley 2 Madras Farm Eqipments Agricultural implements, Cage Mob: 9443521489 Address: No.3, GST Road, wheel, Diesel engines, Heavy Web: Madurantakam - 603 306 duty adjustable tiller, Medium http://www.shrachi.c duty tiller, Power operated om/btl_agro/index. reaper, Power tiller 3 Sri Vinayaga tillers Agricultural implements, Cage Mob: 9944125848 Address: wheel, Diesel engines, Heavy Web: MapuramVillage,Vandavasi duty adjustable tiller, Medium http://www.shrachi.c Road Madurantakam (tk) duty tiller, Power operated om/btl_agro/index.p Kanchipuram reaper, Power tiller hp 4 Tamil Nadu Agro Service Diesel engines, Power tiller Mob: 9842630121, Address: No. 13, East Raja 9842652543. Street, Kanchipuram 5 M/S. Sri Venkateshwara Power tiller Mob: 9443540227 Power Tiller Agencies Address: No. 5, Bus Stand, At P.O Thirukalukundram – 603 109, Dist- Kanchipuram, 6 Bhavani Agro Service Power operated reaper, Power Ph:41112223104 Address: 103,Vanigar Street, tiller Mob: 9894615088 Kanchipuram 7 Ayyan Agro Services Brush cutter, Power tiller, Rice Mob: 9944725059 Address: 10/140 Periya transplanter TheruTirukulam T.K, Kanchipuram 8 Aiswarya Agro Service Brush cutter, Power tiller, Rice Mob: 9944725059 Address:38,B/1 Salai Street transplanter kanchipuram 9 Need Services Chaff cutter Mob: 9443069453 Address: Plot 14, Ayyan Karunai Appar Street, Saravana Avenue, Kannivakkam, Kanchiuram 10 Arasu Agro Services Cage wheel, Cultivator, Disc Mob: 9445393864 Address: shop.no.4, plough Aadhiparasakthi commercial complex, G.S.T. -
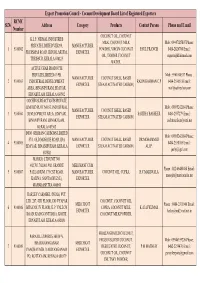
Exporters List
Export Promotion Council - Coconut Development Board List of Registered Exporters RCMC Sl.No Address Category Products Contact Person Phone and E mail Number COCONUT OIL, COCONUT K.L.F. NIRMAL INDUSTRIES MILK, COCONUT MILK Mob : 09447025807 Phone : PRIVATE LIMITED VIII/295, MANUFACTURER 1 9100002 POWDER, VIRGIN COCONUT PAUL FRANCIS 0480-2826704 Email : FR.DISMAS ROAD, IRINJALAKUDA, EXPORTER OIL, TENDER COCONUT [email protected] THRISSUR, KERALA 680125 WATER, ACTIVE CHAR PRODUCTS PRIVATE LIMITED 63/9B, Mob : 9961000337 Phone : MANUFACTURER COCONUT SHELL BASED 2 9100003 INDUSTRIAL DEVELOPMENT RAZIN RAHMAN C.P 0484-2556518 Email : EXPORTER STEAM ACTIVATED CARBON, AREA, BINANIPURAM, EDAYAR, [email protected] ERNAKULAM, KERALA 683502 COCHIN SURFACTANTS PRIVATE LIMITED PLOT NO.63, INDUSTRIAL Mob : 09895242184 Phone : MANUFACTURER COCONUT SHELL BASED 3 9100004 DEVELOPMENT AREA, EDAYAR, SAJITHA BASHEER 0484-2557279 Email : EXPORTER STEAM ACTIVATED CARBON, BINANIPURAM, ERNAKULAM, [email protected] KERALA 683502 INDO GERMAN CARBONS LIMITED Mob : 09895242184 Phone : 57/3, OLD MOSQUE ROAD, IDA MANUFACTURER COCONUT SHELL BASED DR.MOHAMMED 4 9100005 0484-2558105 Email : EDAYAR, BINANIPURAM, KERALA EXPORTER STEAM ACTIVATED CARBON, ALI.P. [email protected] 683502 MARICO LTD UNIT NO. 402,701,702,801,902, GRANDE MERCHANT CUM Phone : 022-66480480 Email : 5 9100007 PALLADIUM, 175 CST ROAD, MANUFACTURER COCONUT OIL, COPRA, H.C MARIWALA [email protected] KALINA, SANTACRUZ (E), EXPORTER MAHARASHTRA 400098 HARLEY CARMBEL (INDIA) PVT. LTD. 287,