2 the Biology of Ageing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Ageing Haematopoietic Stem Cell Compartment
REVIEWS The ageing haematopoietic stem cell compartment Hartmut Geiger1,2, Gerald de Haan3 and M. Carolina Florian1 Abstract | Stem cell ageing underlies the ageing of tissues, especially those with a high cellular turnover. There is growing evidence that the ageing of the immune system is initiated at the very top of the haematopoietic hierarchy and that the ageing of haematopoietic stem cells (HSCs) directly contributes to changes in the immune system, referred to as immunosenescence. In this Review, we summarize the phenotypes of ageing HSCs and discuss how the cell-intrinsic and cell-extrinsic mechanisms of HSC ageing might promote immunosenescence. Stem cell ageing has long been considered to be irreversible. However, recent findings indicate that several molecular pathways could be targeted to rejuvenate HSCs and thus to reverse some aspects of immunosenescence. HSC niche The current demographic shift towards an ageing popu- The innate immune system is also affected by ageing. A specialized lation is an unprecedented global phenomenon that has Although an increase in the number of myeloid precur- microenvironment that profound implications. Ageing is associated with tissue sors has been described in the bone marrow of elderly interacts with haematopoietic attrition and an increased incidence of many types of can- people, the oxidative burst and the phagocytic capacity of stem cells (HSCs) to regulate cers, including both myeloid and lymphoid leukaemias, and both macrophages and neutrophils are decreased in these their fate. other haematopoietic cell malignancies1,2. Thus, we need individuals12,13. Moreover, the levels of soluble immune to understand the molecular and cellular mechanisms of mediators are altered with ageing. -

Biogerontology: a Novel Tool to Stay Healthy in Old Age
MoPAct Policy Brief: 5 Biogerontology: a novel tool to stay healthy in old age Policy priority Healthy lifestyle interventions in particular regarding nutrition and vaccination need to be implemented early in life with a lifecourse perspective. Summary: Key findings: • Accumulating evidence from experimental studies shows that aging is not inevitably linked with the development of chronic diseases. • Only 20-25% of healthy life expectancy (HLE) is predetermined by genes; lifestyle and environment play a major role. • Age-associated accumulation of molecular and cellular damage can be prevented or greatly delayed by lifestyle interventions e.g. dietary manipulations. • Classical strategies (e.g. nutrition, exercise, vaccination) require broad communication to public. • Novel strategies (e.g. dietary interventions, novel drugs, stem cells) need successful translation from the understanding of molecular mechanism to animal models to clinic. • To be successful interventions need to be started early Figure 1. Strategies to increase HLE. (1) Classical interventions include nutrition, exercise, vaccination, no smoking/alcohol/drugs. (2) Novel interventions include in life with a life-course perspective. dietary interventions, clearance of senescent and damaged cells, mitohormetics, stem cells, drugs against inflammation, rejuvenation factors from blood, telomeres, Background: epigenetic drugs, chaperons and proteolytic systems (novel interventions adapted from López-Otín et al., Cell 2013). Population aging is progressing fast in all developed countries. Aging is associated with the development of multiple serious chronic illnesses, including type 2 diabetes, hypertension, heart Prevention disease, stroke, cancer, cognitive impairment and increased Prevention prior to the development of age-associated diseases morbidity and mortality from infectious diseases. As people is key for successful aging. -

Symposium on Aging June 19, 2015 Symposium on Aging the Paul F
The 2015 Harvard / Paul F. Glenn Symposium on Aging June 19, 2015 Symposium on Aging The Paul F. Glenn Laboratories for the Biological Mechanisms of Aging Agenda 10th Anniversary Symposium Welcome to the 10th Annual Harvard/Paul F. Glenn Symposium on Aging. June 19, 2015 Each year, the Paul F. Glenn Laboratories host the Harvard Symposium 9:00 - 5:00 on Aging with a mission to present new advances in aging research and to stimulate collaborative research in this area. The symposium has grown over the past 10 years to be one of the biggest events at Harvard Medical School. We have been fortunate to have many of the leaders in the aging field speak 9:00 – 9:45 a.m. Introduction at the symposia and today is no exception. We wish to acknowledge the generosity and vision of Paul F. Glenn, Mark 9:45 – 10:15 a.m. Ensuring the Health of the Germ-line Collins and Leonard Judson for their unwavering support of aging research Cynthia Kenyon, Ph.D. through the Paul F. Glenn Foundation. Thanks to their support, we now have a vibrant community of researchers who study aging and age-related 10:15 – 10:45 a.m. C. elegans Surveillance of Core Cellular diseases. Since the inception of the Paul F. Glenn labs at Harvard in 2005, Components to Detect and Defend Pathogen this network has grown to include aging studies at the Albert Einstein Attacks, and How This Relates to Longevity College of Medicine, the Buck Institute, the Mayo Clinic, Massachusetts Gary Ruvkun, Ph.D. -

David Sinclair Can Dramatically Extend the Life Span of Yeast
News Focus Lenny Guarente and his former postdoc David Sinclair can dramatically extend the life span of yeast. They’re battling over how this works, and competing head-to-head to grant extra years to humans Aging Research’s Family Feud BOSTON AND CAMBRIDGE,MASSACHUSETTS—At tenure-track spot on Harvard’s faculty in late share many traits common among successful 34, David Sinclair is a rising star. His spa- 1999. He then made clear that his old profes- scientists. Both are deeply ambitious, relent- cious ninth-floor office at Harvard Medical sor’s pet theories weren’t off limits. At a lessly competitive, and supremely self-confi- School boasts a panoramic Boston view. His meeting at Cold Spring Harbor Laboratory dent. Both savor the limelight. Both love sci- rapidly growing lab pulled off the feat of pub- in late 2002, Sinclair surprised Guarente by ence and show little interest in other pursuits. lishing in both Nature and Science last year, challenging him on how a key gene Guarente Still, they’ve retained something of the and it made headlines around the world with discovered extends life in yeast. That sparked parent-child relationship that can shape in- a study of the possible antiaging properties of a bitter dispute that crescendoed this winter, teractions between senior researchers and a molecule found in red wine. In a typical when the pair published dueling papers. their students. Like a father dismayed when day, he fields calls from a couple practicing a Researchers who study aging are finding his son joins a punk rock band, and then dis- radical diet to extend life span, and from an the quarrel both intellectually provocative and mayed further when it attracts devoted fans actor hunting for antiaging pills and the a lively source of gossip. -

Genes That Act Downstream of DAF-16 to Influence the Lifespan Of
articles Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans Coleen T. Murphy*, Steven A. McCarroll†, Cornelia I. Bargmann†, Andrew Fraser‡, Ravi S. Kamath‡, Julie Ahringer‡, Hao Li* & Cynthia Kenyon* * Department of Biochemistry and Biophysics, and † Department of Anatomy and Howard Hughes Medical Institute, University of California, San Francisco, California 94143-2200, USA ‡ Wellcome CRC Institute and Department of Genetics, University of Cambridge, Tennis Court Road, Cambridge CB2 1QR, UK ........................................................................................................................................................................................................................... Ageing is a fundamental, unsolved mystery in biology. DAF-16, a FOXO-family transcription factor, influences the rate of ageing of Caenorhabditis elegans in response to insulin/insulin-like growth factor 1 (IGF-I) signalling. Using DNA microarray analysis, we have found that DAF-16 affects expression of a set of genes during early adulthood, the time at which this pathway is known to control ageing. Here we find that many of these genes influence the ageing process. The insulin/IGF-I pathway functions cell non- autonomously to regulate lifespan, and our findings suggest that it signals other cells, at least in part, by feedback regulation of an insulin/IGF-I homologue. Furthermore, our findings suggest that the insulin/IGF-I pathway ultimately exerts its effect on lifespan by upregulating a wide variety of genes, including cellular stress-response, antimicrobial and metabolic genes, and by downregulating specific life-shortening genes. The recent discovery that the ageing process is regulated hormonally never been tested directly; for example, by asking whether stress by an evolutionarily conserved insulin/IGF-I signalling pathway1–3 response genes are required for the longevity of daf-2 mutants. -

From Here to Immortality: Anti-Aging Medicine
FromFrom HereHere toto Immortality:Immortaalitty: AAnti-AgingAnnntti-AAgging MMedicineedicine Anti-aging medicine is a $5 billion industry. Despite its critics, researchers are discovering that inter ventions designed to turn back time may prove to be more science than fiction. By Trudie Mitschang 14 BioSupply Trends Quarterly • October 2013 he symptoms are disturbing. Weight gain, muscle Shifting Attitudes Fuel a Booming Industry aches, fatigue and joint stiffness. Some experience The notion that aging requires treatment is based on a belief Thear ing loss and diminished eyesight. In time, both that becoming old is both undesirable and unattractive. In the memory and libido will lapse, while sagging skin and inconti - last several decades, aging has become synonymous with nence may also become problematic. It is a malady that begins dete rioration, while youth is increasingly revered and in one’s late 40 s, and currently 100 percent of baby boomers admired. Anti-aging medicine is a relatively new but thriving suffer from it. No one is immune and left untreated ; it always field driven by a baby- boomer generation fighting to preserve leads to death. A frightening new disease, virus or plague? No , its “forever young” façade. According to the market research it’s simply a fact of life , and it’s called aging. firm Global Industry Analysts, the boomer-fueled consumer The mythical fountain of youth has long been the subject of base will push the U.S. market for anti-aging products from folklore, and although it is both natural and inevitable, human about $80 billion now to more than $114 billion by 2015. -

Metabolic Theories of Aging
Metabolic Theories of Aging Chris Burley, Lisa K. Lashley, Charles J. Golden Nova Southeastern University Metabolic theories of aging postulate that aging is due to energy expenditure, which ultimately results in the breakdown and eventual death of cells. Historically, within the realm of metabolic theories of aging, there have been three models: The Rate-of-Living theory, The Oxidative Damage/Free Radical theory, and the Metabolic Stability theory. All three theories maintain that aging is directly related to metabolic rate, but the theories differ in how they arrive at that conclusion. The Rate-of Living theory of aging is the most antiquated of the metabolic aging theories. It proposes that two factors are responsible for determining longevity in all living organisms: 1) a genetically pre-determined capacity for overall metabolic potential, and 2) metabolic rate. The first empirical evidence for this theory dates back to 1908, when Max Rubner observed increasing metabolic rates as a function of increasing body mass in five mammalian species of varying longevities (guinea pig, cat, dog, cow, and horse). Rubner reported that the animals’ metabolic rates increased as a function of body mass, and that larger animals had longer lifespans. Based on these results, Rubner postulated that longevity could be determined by calculating the pre-determined capacity for organic energy use and the rate at which the energy was being expended. Rubner’s observation was supported by Raymond Pearl when he concluded that slower metabolism resulted in an increased lifespan among fruit flies and cantaloupe seeds. Despite the evidence for the Rate-of-Living theory, modern scientific developments have refuted the accuracy of the theory. -

Forkhead Transcription Factors and Ageing
Oncogene (2008) 27, 2351–2363 & 2008 Nature Publishing Group All rights reserved 0950-9232/08 $30.00 www.nature.com/onc REVIEW Forkhead transcription factors and ageing L Partridge1 and JC Bru¨ ning2 1Institute of Healthy Ageing, GEE, London, UK; 2Department of Mouse Genetics and Metabolism, Institute for Genetics University of Cologne, Cologne, Germany Mutations in single genes and environmental interventions Forkhead transcription factors are turning out to play can extend healthy lifespan in laboratory model organi- a key role in invertebrate models ofextension ofhealthy sms. Some of the mechanisms involved show evolutionary lifespan by single-gene mutations, and evidence is conservation, opening the way to using simpler inverte- mounting for their importance in mammals. Forkheads brates to understand human ageing. Forkhead transcrip- can also play a role in extension oflifespanby dietary tion factors have been found to play a key role in lifespan restriction, an environmental intervention that also extension by alterations in the insulin/IGF pathway and extends lifespan in diverse organisms (Kennedy et al., by dietary restriction. Interventions that extend lifespan 2007). Here, we discuss these findings and their have also been found to delay or ameliorate the impact of implications. The forkhead family of transcription ageing-related pathology and disease, including cancer. factors is characterized by a type of DNA-binding Understanding the mode of action of forkheads in this domain known as the forkhead box (FOX) (Weigel and context will illuminate the mechanisms by which ageing Jackle, 1990). They are also called winged helix acts as a risk factor for ageing-related disease, and could transcription factors because of the crystal structure lead to the development of a broad-spectrum, preventative ofthe FOX, ofwhich the forkheadscontain a medicine for the diseases of ageing. -

World Population Ageing 2019
World Population Ageing 2019 Highlights ST/ESA/SER.A/430 Department of Economic and Social Affairs Population Division World Population Ageing 2019 Highlights United Nations New York, 2019 The Department of Economic and Social Affairs of the United Nations Secretariat is a vital interface between global policies in the economic, social and environmental spheres and national action. The Department works in three main interlinked areas: (i) it compiles, generates and analyses a wide range of economic, social and environmental data and information on which States Members of the United Nations draw to review common problems and take stock of policy options; (ii) it facilitates the negotiations of Member States in many intergovernmental bodies on joint courses of action to address ongoing or emerging global challenges; and (iii) it advises interested Governments on the ways and means of translating policy frameworks developed in United Nations conferences and summits into programmes at the country level and, through technical assistance, helps build national capacities. The Population Division of the Department of Economic and Social Affairs provides the international community with timely and accessible population data and analysis of population trends and development outcomes for all countries and areas of the world. To this end, the Division undertakes regular studies of population size and characteristics and of all three components of population change (fertility, mortality and migration). Founded in 1946, the Population Division provides substantive support on population and development issues to the United Nations General Assembly, the Economic and Social Council and the Commission on Population and Development. It also leads or participates in various interagency coordination mechanisms of the United Nations system. -

Shared Ageing Research Models (Sharm): a New Facility to Support Ageing Research
Biogerontology (2013) 14:789–794 DOI 10.1007/s10522-013-9457-0 METHOD Shared Ageing Research Models (ShARM): a new facility to support ageing research Adele L. Duran • Paul Potter • Sara Wells • Tom Kirkwood • Thomas von Zglinicki • Anne McArdle • Cheryl Scudamore • Qing-Jun Meng • Gerald de Haan • Anne Corcoran • Ilaria Bellantuono Received: 5 July 2013 / Accepted: 16 August 2013 / Published online: 2 October 2013 Ó The Author(s) 2013. This article is published with open access at Springerlink.com Abstract In order to manage the rise in life expec- Wellcome Trust, open to all investigators. It collects, tancy and the concomitant increased occurrence of stores and distributes flash frozen tissues from aged age-related diseases, research into ageing has become murine models through its biorepository and provides a strategic priority. Mouse models are commonly a database of live ageing mouse colonies available in utilised as they share high homology with humans and the UK and abroad. It also has an online environment show many similar signs and diseases of ageing. (MICEspace) for collation and analysis of data from However, the time and cost needed to rear aged communal models and discussion boards on subjects cohorts can limit research opportunities. Sharing of such as the welfare of ageing animals and common resources can provide an ethically and economically endpoints for intervention studies. Since launching in superior framework to overcome some of these issues July 2012, thanks to the generosity of researchers in but requires dedicated infrastructure. Shared Ageing UK and Europe, ShARM has collected more than Research Models (ShARM) (www.ShARMUK.org) 2,500 tissues and has in excess of 2,000 mice regis- is a new, not-for-profit organisation funded by tered in live ageing colonies. -
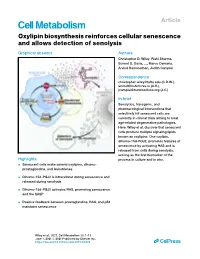
Viewer Comments
Article Oxylipin biosynthesis reinforces cellular senescence and allows detection of senolysis Graphical abstract Authors Christopher D. Wiley, Rishi Sharma, Sonnet S. Davis, ..., Marco Demaria, Arvind Ramanathan, Judith Campisi Correspondence [email protected] (C.D.W.), [email protected] (A.R.), [email protected] (J.C.) In brief Senolytics, transgenic, and pharmacological interventions that selectively kill senescent cells are currently in clinical trials aiming to treat age-related degenerative pathologies. Here, Wiley et al. discover that senescent cells produce multiple signaling lipids known as oxylipins. One oxylipin, dihomo-15d-PGJ2, promotes features of senescence by activating RAS and is released from cells during senolysis, serving as the first biomarker of the Highlights process in culture and in vivo. d Senescent cells make several oxylipins, dihomo- prostaglandins, and leukotrienes d Dihomo-15d-PGJ2 is intracellular during senescence and released during senolysis d Dihomo-15d-PGJ2 activates RAS, promoting senescence and the SASP d Positive feedback between prostaglandins, RAS, and p53 maintains senescence Wiley et al., 2021, Cell Metabolism 33, 1–13 June 1, 2021 ª 2021 Published by Elsevier Inc. https://doi.org/10.1016/j.cmet.2021.03.008 ll Please cite this article in press as: Wiley et al., Oxylipin biosynthesis reinforces cellular senescence and allows detection of senolysis, Cell Metabolism (2021), https://doi.org/10.1016/j.cmet.2021.03.008 ll Article Oxylipin biosynthesis reinforces cellular senescence and allows detection of senolysis Christopher D. Wiley,1,2,* Rishi Sharma,1 Sonnet S. Davis,1 Jose Alberto Lopez-Dominguez,1 Kylie P. Mitchell,1 Samantha Wiley,1 Fatouma Alimirah,1 Dong Eun Kim,1 Therese Payne,1 Andrew Rosko,1 Eliezer Aimontche,1 Sharvari M. -

HCB 524 — Transhumanism
HCB 524 Special Topic in Bioethics Fall Semester, 2019. Tuesdays 6-8:30pm. Instructor of Record: Adam Sepe, MA, MLS(ASCP)cm [email protected] Course Description: Transhumanism and [Human?] Dignity. Throughout human history — and prehistory for that matter — technological advancement has drastically altered every aspect of human life. Most of us will say that many advents — such as cooking and the wheel — have been largely, if not entirely, beneficial. Would we say the same of all technology? Surely each of us can list technologies that have, in the very least, some considerable downsides. So while history and experience can tell us that some technologies are beneficial and that some other technologies are harmful, how can we know what kind of impact future technology will have? For now we can’t, and so all we can do is try, to the best of our ability, to imagine such futures and develop our technology with these considerations in mind. ‘Transhumanism’ refers a diverse collection of ideas that have one at least thing in common: through future technology, humanity will be fundamentally altered to an unprecedented degree. Some even believe there will come a time when, through our own action, the word ‘human’ will be obsolete; that we will be succeeded by entities (or an entity) for which ‘human’ does not apply. Most people who identify as transhumanists are, to varying degrees, proponents of such technology. They are in favor of such alterations and they argue that these will be beneficial. In this course, we will take a critical look at transhumanist claims.