Strengthening Marine Amphipod DNA Barcode Libraries for Environmental Monitoring
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Complete Transcriptome Assembly and Annotation of a Critically Important
Environment International 137 (2020) 105319 Contents lists available at ScienceDirect Environment International journal homepage: www.elsevier.com/locate/envint Complete transcriptome assembly and annotation of a critically important amphipod species in freshwater ecotoxicological risk assessment: Gammarus T fossarum ⁎ Domenico R. Caputoa,1, Samuel C. Robsonb,1, Inge Wernerc, Alex T. Forda, a Institute of Marine Sciences, School of Biological Sciences, University of Portsmouth, Ferry Road, Portsmouth PO4 9LY, UK b Centre for Enzyme Innovation, St. Michael's Building, University of Portsmouth, White Swan Road, Portsmouth PO1 2DT, UK c Swiss Centre for Applied Ecotoxicology, Eawag - EPFL, Überlandstrasse 133, 8600 Dübendorf, Switzerland ARTICLE INFO ABSTRACT Handling Editor: Hefa Cheng Because of their crucial role in ecotoxicological risk assessment, amphipods (Crustacea) are commonly employed Keywords: as model species in a wide range of studies. However, despite their ecological importance, their genome has not Gammarus fossarum yet been completely annotated and molecular mechanisms underlying key pathways, such as the serotonin RNA sequencing pathway, in development of ecotoxicological biomarkers of exposure to neuroactive pharmaceuticals are still De novo assembly poorly understood. Furthermore, genetic similarities and discrepancies with other model arthropods (e.g., Serotonin pathway Drosophila melanogaster) have not been completely clarified. In this report, we present a new transcriptome assembly of Gammarus fossarum, an important amphipod species, widespread in Central Europe. RNA-Seq with Illumina HiSeq technology was used to analyse samples extracted from total internal tissues. We used the Trinity and Trinotate software suites for transcriptome assembly and annotation, respectively. The quality of this as- sembly and the affiliated targeted homology searches greatly enrich the molecular knowledge on this species. -

Phylogeny and Phylogeography of the Family Hyalidae (Crustacea: Amphipoda) Along the Northeast Atlantic Coasts
ALMA MATER STUDIORUM UNIVERSITÀ DI BOLOGNA SCUOLA DI SCIENZE - CAMPUS DI RAVENNA CORSO DI LAUREA MAGISTRALE IN BIOLOGIA MARINA Phylogeny and phylogeography of the family Hyalidae (Crustacea: Amphipoda) along the northeast Atlantic coasts Tesi di laurea in Alterazione e Conservazione degli Habitat Marini Relatore Presentata da Prof. Marco Abbiati Andrea Desiderato Correlatore Prof. Henrique Queiroga II sessione Anno accademico 2014/2015 “...Nothing at first can appear more difficult to believe than that the more complex organs and instincts should have been perfected, not by means superior to, though analogous with, human reason, but by the accumulation of innumerable slight variations, each good for the individual possessor…” (Darwin 1859) 1 1) Index 1) Index ------------------------------------------------------------------------------------------------ 2 2) Abstract ------------------------------------------------------------------------------------------- 3 3) Introduction ------------------------------------------------------------------------------------- 4 a) Hyalidae Bulycheva, 1957 ----------------------------------------------------------------- 4 b) Phylogeny -------------------------------------------------------------------------------------- 6 i) Phylogeny of Hyalidae -------------------------------------------------------------------- 7 c) The DNA barcode --------------------------------------------------------------------------- 8 d) Apohyale prevostii (Milne Edwars, 1830) --------------------------------------------- 9 -

Guide to Crustacea
38 Guide to Crustacea. This is a very large and varied group, comprising numerous families which are grouped under six Sub-orders. In the Sub-order ASELLOTA the uropods are slender ; the basal segments of the legs are not coalesced with the body as in most other Isopoda ; the first pair of abdominal limbs are generally fused, in the female, to form an operculum, or cover for the remaining pairs. This group includes Asellus aquaticus, which is FIG. 23. Bathynomus giganteus, about one-half natural size. (From Lankester's "Treatise on Zoology," after Milne-Edwards and Bouvier.) [Table-case No. 6.] common everywhere in ponds and ditches in this country, and a very large number of marine species, mostly of small size. The Sub-order PHKEATOICIDEA includes a small number of very peculiar species found in fresh water in Australia and New Zealand. In these the body is flattened from side to side, and Peraca rida—Isopoda. 39 the animals in other respects have a superficial resemblance to Amphipoda. In the Sub-order FLABELLIFERA the terminal limbs of the abdomen (uropods) are spread out in a fan-like manner on each side of the telson. Many species of this group, belonging to the family Cymothoidae, are blood-sucking parasites of fish, and some of them are remarkable for being hermaphrodite (like the Cirri- pedia), each animal being at first a male and afterwards a female. Mo' of these parasites are found adhering to the surface of the body, behind the fins or under the gill-covers of the fish. A few, however, become internal parasites like the Artystone trysibia exhibited in this case, which has burrowed into the body of a Brazilian freshwater fish. -

Additions to and Revisions of the Amphipod (Crustacea: Amphipoda) Fauna of South Africa, with a List of Currently Known Species from the Region
Additions to and revisions of the amphipod (Crustacea: Amphipoda) fauna of South Africa, with a list of currently known species from the region Rebecca Milne Department of Biological Sciences & Marine Research Institute, University of CapeTown, Rondebosch, 7700 South Africa & Charles L. Griffiths* Department of Biological Sciences & Marine Research Institute, University of CapeTown, Rondebosch, 7700 South Africa E-mail: [email protected] (with 13 figures) Received 25 June 2013. Accepted 23 August 2013 Three species of marine Amphipoda, Peramphithoe africana, Varohios serratus and Ceradocus isimangaliso, are described as new to science and an additional 13 species are recorded from South Africa for the first time. Twelve of these new records originate from collecting expeditions to Sodwana Bay in northern KwaZulu-Natal, while one is an introduced species newly recorded from Simon’s Town Harbour. In addition, we collate all additions and revisions to the regional amphipod fauna that have taken place since the last major monographs of each group and produce a comprehensive, updated faunal list for the region. A total of 483 amphipod species are currently recognized from continental South Africa and its Exclusive Economic Zone . Of these, 35 are restricted to freshwater habitats, seven are terrestrial forms, and the remainder either marine or estuarine. The fauna includes 117 members of the suborder Corophiidea, 260 of the suborder Gammaridea, 105 of the suborder Hyperiidea and a single described representative of the suborder Ingolfiellidea. -

ANSWERED ON:11.05.2005 AUTOMATIC and MODERN TELEPHONE EXCHANGES in TAMIL NADU Kharventhan Shri Salarapatty Kuppusamy
GOVERNMENT OF INDIA COMMUNICATIONS AND INFORMATION TECHNOLOGY LOK SABHA UNSTARRED QUESTION NO:6879 ANSWERED ON:11.05.2005 AUTOMATIC AND MODERN TELEPHONE EXCHANGES IN TAMIL NADU Kharventhan Shri Salarapatty Kuppusamy Will the Minister of COMMUNICATIONS AND INFORMATION TECHNOLOGY be pleased to state: (a) the details of automatic and modern telephone exchanges set up in Tamil Nadu during the last three years, location- wise; (b) the details of such exchanges proposed to be set up in Tamil Nadu during the current year; (c) the details of the telephone exchanges whose capacities were expanded in the current financial year; and (d) the details of telephone exchanges where waiting list for telephone connection still exists? Answer THE MINISTER OF STATE IN THE MINISTRY OF COMMUNICATIONS ANDINFORMATION TECHNOLOGY (DR. SHAKEEL AHMAD) (a) The details of automatic and modern telephone exchanges set up in Tamilnadu during the last three years are given in the Annexures- I(a), I(b) & I(c). (b) The details of such exchanges proposed to be set up in Tamilnadu during the current year are given in Annexure-II. (c) The details of the telephone exchanges whose capacities were expanded in the current financial year are given at Annexure-III. (d) The details of telephone exchanges where waiting list for telephone connection still exists are given in Annexure- IV. ANNEXURE-I(a) DETAILS OF TELEPHONE EXCHANGES SET UP DURING 2002-03 IN TAMILNADU Sl Name of Exchange Capacity Type/Technology District No.(Location) 1 Avinashi-II 4000 CDOTMBMXL Coimbatore 2 K.P.Pudur -

Crustacea: Amphipoda: Dogielinotidae) from the Nipa Palm in Thailand, with an Updated Key to the Genera
RESEARCH ARTICLE Discovery of a new genus and species of dogielinotid amphipod (Crustacea: Amphipoda: Dogielinotidae) from the Nipa palm in Thailand, with an updated key to the genera 1,2 3 4 Koraon WongkamhaengID *, Pongrat Dumrongrojwattana , Myung-Hwa Shin a1111111111 a1111111111 1 Department of Zoology, Faculty of Science, Kasetsart University, Bangkok, Thailand, 2 Coastal Oceanography and Climate Change Research Center, Prince of Songkla University, Hatyai, Songkhla, a1111111111 Thailand, 3 Department of Biology, Faculty of Science, Burapha University, Bangsaen, Chonburi, Thailand, a1111111111 4 National Marine Biodiversity Institute of Korea, Seocheon, South Korea a1111111111 * [email protected] Abstract OPEN ACCESS Citation: Wongkamhaeng K, Dumrongrojwattana During a scientific survey, a new genus of the dogielinotid amphipoda was found in the Nipa P, Shin M (2018) Discovery of a new genus and palm (Nypa fruticans) in Bang Krachao Urban Oasis, Samut Prakan Province, Thailand. We species of dogielinotid amphipod (Crustacea: placed this new genus, Allorchestoides gen. nov., within the family Dogielinotidae. The new Amphipoda: Dogielinotidae) from the Nipa palm in Thailand, with an updated key to the genera. PLoS taxa can be easily distinguished from the remaining genera by differences in the incisor of ONE 13(10): e0204299. https://doi.org/10.1371/ the left and right mandibles, apical robust setae of the maxilla 1, and the large coxa and journal.pone.0204299 strong obtuse palm in the female gnathopod 1. The type species of Allorchestoides gen. Editor: Feng Zhang, Nanjing Agricultural University, nov., Allorchestoides rosea n. sp., is described here in, with an updated key to the genera of CHINA the family Dogielinotidae. -

The 17Th International Colloquium on Amphipoda
Biodiversity Journal, 2017, 8 (2): 391–394 MONOGRAPH The 17th International Colloquium on Amphipoda Sabrina Lo Brutto1,2,*, Eugenia Schimmenti1 & Davide Iaciofano1 1Dept. STEBICEF, Section of Animal Biology, via Archirafi 18, Palermo, University of Palermo, Italy 2Museum of Zoology “Doderlein”, SIMUA, via Archirafi 16, University of Palermo, Italy *Corresponding author, email: [email protected] th th ABSTRACT The 17 International Colloquium on Amphipoda (17 ICA) has been organized by the University of Palermo (Sicily, Italy), and took place in Trapani, 4-7 September 2017. All the contributions have been published in the present monograph and include a wide range of topics. KEY WORDS International Colloquium on Amphipoda; ICA; Amphipoda. Received 30.04.2017; accepted 31.05.2017; printed 30.06.2017 Proceedings of the 17th International Colloquium on Amphipoda (17th ICA), September 4th-7th 2017, Trapani (Italy) The first International Colloquium on Amphi- Poland, Turkey, Norway, Brazil and Canada within poda was held in Verona in 1969, as a simple meet- the Scientific Committee: ing of specialists interested in the Systematics of Sabrina Lo Brutto (Coordinator) - University of Gammarus and Niphargus. Palermo, Italy Now, after 48 years, the Colloquium reached the Elvira De Matthaeis - University La Sapienza, 17th edition, held at the “Polo Territoriale della Italy Provincia di Trapani”, a site of the University of Felicita Scapini - University of Firenze, Italy Palermo, in Italy; and for the second time in Sicily Alberto Ugolini - University of Firenze, Italy (Lo Brutto et al., 2013). Maria Beatrice Scipione - Stazione Zoologica The Organizing and Scientific Committees were Anton Dohrn, Italy composed by people from different countries. -

Tamil Nadu Government Gazette
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2018 [Price: Rs. 20.00 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 31] CHENNAI, WEDNESDAY, AUGUST 1, 2018 Aadi 16, Vilambi, Thiruvalluvar Aandu – 2049 Part VI—Section 4 Advertisements by private individuals and private institutions CONTENTS PRIVATE ADVERTISEMENTS Pages. Change of Names .. 1279-1328 Notice .. NOTICE NO LEGAL RESPONSIBILITY IS ACCEPTED FOR THE PUBLICATION OF ADVERTISEMENTS REGARDING CHANGE OF NAME IN THE TAMIL NADU GOVERNMENT GAZETTE. PERSONS NOTIFYING THE CHANGES WILL REMAIN SOLELY RESPONSIBLE FOR THE LEGAL CONSEQUENCES AND ALSO FOR ANY OTHER MISREPRESENTATION, ETC. (By Order) Director of Stationery and Printing. CHANGE OF NAMES 18547. I, S. Muminal, wife of Thiru R. Syed Mohamed 18550. My son, Santhanagurus, born on 11th April 2006 Mahdoom, born on 23rd June 1981 (native district: (native district: Virudhunagar), residing at No. 123-B, Sivagangai), residing at No. 518, Sathapuli, Kollankudi, Santhaipettai Street, Srivilliputtur Taluk, Virudhunagar- Alagapuri Post, Sivagangai-630 556, shall henceforth be 626 125, shall henceforth be known as L. FELIX PERNANDO known as S. SAYED MOOMINAL L.T.I. of R. LURDU MICHAL S. MUMINAL Virudhunagar, 23rd July 2018. (Father) Sivagangai, 23rd July 2018. 18551. I, D. Jamila Begam, wife of Thiru A. Dhivan Sha, 18548. My son, Sereesha Habib, born on 16th July 2004 born on 4th July 1991 (native district: Madurai), residing at (native district: Sivagangai), residing at No. 518, No. 70, Valluvar South Street, Jeeva Nagar 1st Street, Sathapuli, Kollankudi, Alagapuri Post, Sivagangai-630 556, Jaihindpuram, Madurai-625 011, shall henceforth be shall henceforth be known as S. -
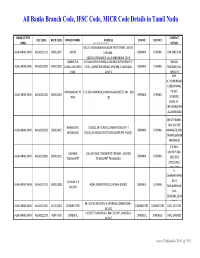
Banks Branch Code, IFSC Code, MICR Code Details in Tamil Nadu
All Banks Branch Code, IFSC Code, MICR Code Details in Tamil Nadu NAME OF THE CONTACT IFSC CODE MICR CODE BRANCH NAME ADDRESS CENTRE DISTRICT BANK www.Padasalai.Net DETAILS NO.19, PADMANABHA NAGAR FIRST STREET, ADYAR, ALLAHABAD BANK ALLA0211103 600010007 ADYAR CHENNAI - CHENNAI CHENNAI 044 24917036 600020,[email protected] AMBATTUR VIJAYALAKSHMIPURAM, 4A MURUGAPPA READY ST. BALRAJ, ALLAHABAD BANK ALLA0211909 600010012 VIJAYALAKSHMIPU EXTN., AMBATTUR VENKATAPURAM, TAMILNADU CHENNAI CHENNAI SHANKAR,044- RAM 600053 28546272 SHRI. N.CHANDRAMO ULEESWARAN, ANNANAGAR,CHE E-4, 3RD MAIN ROAD,ANNANAGAR (WEST),PIN - 600 PH NO : ALLAHABAD BANK ALLA0211042 600010004 CHENNAI CHENNAI NNAI 102 26263882, EMAIL ID : CHEANNA@CHE .ALLAHABADBA NK.CO.IN MR.ATHIRAMIL AKU K (CHIEF BANGALORE 1540/22,39 E-CROSS,22 MAIN ROAD,4TH T ALLAHABAD BANK ALLA0211819 560010005 CHENNAI CHENNAI MANAGER), MR. JAYANAGAR BLOCK,JAYANAGAR DIST-BANGLAORE,PIN- 560041 SWAINE(SENIOR MANAGER) C N RAVI, CHENNAI 144 GA ROAD,TONDIARPET CHENNAI - 600 081 MURTHY,044- ALLAHABAD BANK ALLA0211881 600010011 CHENNAI CHENNAI TONDIARPET TONDIARPET TAMILNADU 28522093 /28513081 / 28411083 S. SWAMINATHAN CHENNAI V P ,DR. K. ALLAHABAD BANK ALLA0211291 600010008 40/41,MOUNT ROAD,CHENNAI-600002 CHENNAI CHENNAI COLONY TAMINARASAN, 044- 28585641,2854 9262 98, MECRICAR ROAD, R.S.PURAM, COIMBATORE - ALLAHABAD BANK ALLA0210384 641010002 COIIMBATORE COIMBATORE COIMBOTORE 0422 2472333 641002 H1/H2 57 MAIN ROAD, RM COLONY , DINDIGUL- ALLAHABAD BANK ALLA0212319 NON MICR DINDIGUL DINDIGUL DINDIGUL -
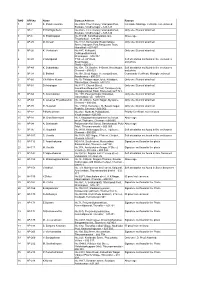
SNO APP.No Name Contact Address Reason 1 AP-1 K
SNO APP.No Name Contact Address Reason 1 AP-1 K. Pandeeswaran No.2/545, Then Colony, Vilampatti Post, Intercaste Marriage certificate not enclosed Sivakasi, Virudhunagar – 626 124 2 AP-2 P. Karthigai Selvi No.2/545, Then Colony, Vilampatti Post, Only one ID proof attached. Sivakasi, Virudhunagar – 626 124 3 AP-8 N. Esakkiappan No.37/45E, Nandhagopalapuram, Above age Thoothukudi – 628 002. 4 AP-25 M. Dinesh No.4/133, Kothamalai Road,Vadaku Only one ID proof attached. Street,Vadugam Post,Rasipuram Taluk, Namakkal – 637 407. 5 AP-26 K. Venkatesh No.4/47, Kettupatti, Only one ID proof attached. Dokkupodhanahalli, Dharmapuri – 636 807. 6 AP-28 P. Manipandi 1stStreet, 24thWard, Self attestation not found in the enclosures Sivaji Nagar, and photo Theni – 625 531. 7 AP-49 K. Sobanbabu No.10/4, T.K.Garden, 3rdStreet, Korukkupet, Self attestation not found in the enclosures Chennai – 600 021. and photo 8 AP-58 S. Barkavi No.168, Sivaji Nagar, Veerampattinam, Community Certificate Wrongly enclosed Pondicherry – 605 007. 9 AP-60 V.A.Kishor Kumar No.19, Thilagar nagar, Ist st, Kaladipet, Only one ID proof attached. Thiruvottiyur, Chennai -600 019 10 AP-61 D.Anbalagan No.8/171, Church Street, Only one ID proof attached. Komathimuthupuram Post, Panaiyoor(via) Changarankovil Taluk, Tirunelveli, 627 761. 11 AP-64 S. Arun kannan No. 15D, Poonga Nagar, Kaladipet, Only one ID proof attached. Thiruvottiyur, Ch – 600 019 12 AP-69 K. Lavanya Priyadharshini No, 35, A Block, Nochi Nagar, Mylapore, Only one ID proof attached. Chennai – 600 004 13 AP-70 G. -

Electrophoretic Approach to the Biochemical Systematics of Gammarids
HELGOL.~NDER MEERESUNTERSUCHUNGEN Helgol~inder Meeresunters. 34, 391-400 (1981) Electrophoretic approach to the biochemical systematics of gammarids H.-P. Bulnheim 1 & A. Scholl 2 IBiologische Anstalt Helgoland (Zentrale); Notkestr. 31, D-2000 Hamburg 52, Federal Republic o[ Germany 2Zoologisches Institut der Universit~t; Baltzerstr. 8, CH-3012 Bern, Switzerland ABSTRACT: By utilizing the techniques for electrophoretic separation of proteins by vertical starch gels, the biochemical systematics of 10 Gammaridae species obtained from marine, brackish and freshwater habitats was studied. They included Chaetogammarus marinus, Gammarus zaddachi, G. salinus, G. oceanicus, G. tigrinus, G. chevreuxi, G. locusta, G. duebeni duebeni, G. d. celticus, G. putex pulex, and G. fossorurn. For comparison of electrophoretic mobilities selected enzymes (phosphoglucose isomerase, glutamate oxalacetate transaminase, arginine phosphokinase, hexo- kinase, leucine amino peptidase, mannose 6-phosphate isomerase) were assayed. They were used as diagnostic characters in terms of electrophoretic identities or diversities of most frequent alleles at polymorphic gene loci. These criteria could be applied to estimate intrageneric enzymic variation and degrees of genetic relatedness between the crustacean amphipod species under consideration, thereby complementing traditional morphological classification. INTRODUCTION The Gammaridae include a very large number of species inhabiting marine, brack- ish and fresh-waters, both epigean and hypogean. Their complex systematics has been subject of many studies and is still being treated at specific, generic and other levels of classification. In view of little morphological differentiation detected between several species and considerable variation of the characters used for diagnosis, certain members of the genus Gammarus proved to be a source of confusion for a long period of time. -

Cladistic Revision of Talitroidean Amphipods (Crustacea, Gammaridea), with a Proposal of a New Classification
CladisticBlackwell Publishing, Ltd. revision of talitroidean amphipods (Crustacea, Gammaridea), with a proposal of a new classification CRISTIANA S. SEREJO Accepted: 8 December 2003 Serejo, C. S. (2004). Cladistic revision of talitroidean amphipods (Crustacea, Gammaridea), with a proposal of a new classification. — Zoologica Scripta, 33, 551–586. This paper reports the results of a cladistic analysis of the Talitroidea s.l., which includes about 400 species, in 96 genera distributed in 10 families. The analysis was performed using PAUP and was based on a character matrix of 34 terminal taxa and 43 morphological characters. Four most parsimonious trees were obtained with 175 steps (CI = 0.617, RI = 0.736). A strict consensus tree was calculated and the following general conclusions were reached. The superfamily Talitroidea is elevated herein as infraorder Talitrida, which is subdivided into three main branches: a small clade formed by Kuria and Micropythia (the Kurioidea), and two larger groups maintained as distinct superfamilies (Phliantoidea, including six families, and Talitroidea s.s., including four). Within the Talitroidea s.s., the following taxonomic changes are proposed: Hyalellidae and Najnidae are synonymized with Dogielinotidae, and treated as subfamilies; a new family rank is proposed for the Chiltoniinae. Cristiana S. Serejo, Museu Nacional/UFRJ, Quinta da Boa Vista s/n, 20940–040, Rio de Janeiro, RJ, Brazil. E-mail: [email protected] Introduction Table 1 Talitroidean classification following Barnard & Karaman The talitroideans include amphipods ranging in length from 1991), Bousfield (1996) and Bousfield & Hendrycks (2002) 3 to 30 mm, and are widely distributed in the tropics and subtropics. In marine and estuarine environments, they are Superfamily Talitroidea Rafinesque, 1815 Family Ceinidae Barnard, 1972 usually found in shallow water, intertidally or even in the supra- Family Dogielinotidae Gurjanova, 1953 littoral zone.