NROSCI/BIOSC 1070 and MSNBIO 2070 September 27, 2017
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

24. the Endothelial Glycocalyx (C Alphonsus)
Part I Anaesthesia Refresher Course – 2018 24 University of Cape Town The Endothelial Glycocalyx Dr Christella Alphonsus Dept of Anaesthesia & Perioperative Medicine University of Cape Town Overview The glycocalyx is a complex gel layer that coats all healthy vascular endothelium. This layer exists in dynamic interaction between flowing blood and the endothelial cell wall. It plays a pivotal role in vascular protection, modulation and haemostasis. Components The endothelial glycocalyx is essentially made up of glycoproteins or proteoglycans but there is great diversity in structure and function within these two groups. Proteoglycans have a protein core to which are attached negatively charged glycosaminoglycan (GAG) side chains. There are different types of proteoglycans and they vary according to: the core protein size, the number of GAG side chains and whether they are bound or not bound to the cell membrane. Syndecans and glypicans are first bound to the cell membrane and the GAG side chains are attached later. Other proteoglycans (perlecans, versicans, decorins, biglycans, mimecans) are first bound to GAGs and then secreted by the endothelial cells. There are five types of GAG side chains: heparan sulphate makes up 50–90%, with the remainder composed of hyaluronic acid and chondroiton, dermatan and keratin sulphates. Hyaluronic acid is the only GAG not usually bound to a core protein and forms viscous solutions with water. Glycoproteins act as adhesion molecules and contribute to the coagulation, fibrinolytic and haemostatic systems. These include E and P selectin which are expressed after stimulation by histamine, thrombin, interleukin-1 and tumour necrosis factora (TNF-α). Ligands for leucocyte and platelet adhesion ICAM-1, ICAM-2, VCAM-1, PECAM-1 are also expressed within the glycocalyx. -

Application of Negative Tissue Interstitial Pressure Improves Functional Capillary Density After Hemorrhagic Shock in the Absence of Volume Resuscitation
UC San Diego UC San Diego Previously Published Works Title Application of negative tissue interstitial pressure improves functional capillary density after hemorrhagic shock in the absence of volume resuscitation. Permalink https://escholarship.org/uc/item/3pc6m5m7 Journal Physiological reports, 9(5) ISSN 2051-817X Authors Jani, Vinay P Jani, Vivek P Munoz, Carlos J et al. Publication Date 2021-03-01 DOI 10.14814/phy2.14783 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Received: 21 January 2021 | Accepted: 5 February 2021 DOI: 10.14814/phy2.14783 ORIGINAL ARTICLE Application of negative tissue interstitial pressure improves functional capillary density after hemorrhagic shock in the absence of volume resuscitation Vinay P. Jani1 | Vivek P. Jani2 | Carlos J. Munoz1 | Krianthan Govender1 | Alexander T. Williams1 | Pedro Cabrales1 1Department of Bioengineering, University of California, San Diego, La Abstract Jolla, CA, USA Microvascular fluid exchange is primarily dependent on Starling forces and both the ac- 2 Division of Cardiology, Department of tive and passive myogenic response of arterioles and post-capillary venules. Arterioles Medicine, The Johns Hopkins University, The Johns Hopkins School of Medicine are classically considered resistance vessels, while venules are considered capacitance Baltimore, MD, USA vessels with high distensibility and low tonic sympathetic stimulation at rest. However, few studies have investigated the effects of modulating interstitial hydrostatic pressure, Correspondence Pedro Cabrales, University of particularly in the context of hemorrhagic shock. The objective of this study was to in- California, San Diego Department of vestigate the mechanics of arterioles and functional capillary density (FCD) during appli- Bioengineering, 0412 9500 Gilman cation of negative tissue interstitial pressure after 40% total blood volume hemorrhagic Dr. -

Open Thesis.Pdf
The Pennsylvania State University The Graduate School College of Engineering ROLE OF HEMODYNAMIC FORCES IN SMOOTH MUSCLE CELL CONTRACTION AND TRANSVASCULAR FILTRATION IN VIVO A Thesis in Bioengineering by Min-ho Kim © 2004 Min-ho Kim Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy December 2004 The thesis of Min-ho Kim was reviewed and approved* by the following: Norman R. Harris Associate Professor of Bioengineering Thesis Advisor Chair of Committee Herbert H. Lipowsky Professor of Bioengineering Peter J. Butler Assistant Professor of Bioengineering Donna H. Korzick Assistant Professor of Physiology and Kinesiology John M. Tarbell Distinguished Professor of Biomedical Engineering City College of New York / CUNY Special Member Herbert H. Lipowsky Professor of Bioengineering Head of the Department of Bioengineering *Signatures are on file in the Graduate School iii ABSTRACT The vascular wall is continuously exposed to two hemodynamic forces imparted by blood flow: pressure and shear stress. Significant changes in hemodynamic forces can occur in many physiological and pathophysiological circumstances. Changes in vascular pressure and blood flow-induced shear stress can affect vascular endothelium and smooth muscle cells (SMCs) mechanically. When vascular pressure is increased, an increase in transvascular filtration is driven by a classical Starling mechanism. This increased transvascular filtration is expected to induce increases in shear stresses through the inter- endothelial cleft surfaces and SMCs. A main hypothesis of the current study is that transvascular filtration-induced shear stress might play important roles in endothelial barrier function and SMC contractions. To address this hypothesis, we investigated the effect of pressure-induced transvascular fluid flux on SMC contraction in vivo. -

3 Physical Properties of the Body Fluids and the Cell Membrane
Physical Properties 3 of the Body Fluids and the Cell Membrane 3.1 BODY FluIDS To begin our study of transport phenomena in biomedical engineering, we must first examine the physical properties of the fluids within the human body. Many of our engineering calculations and the development and design of new procedures, devices, and treatments will either involve or affect the fluids within the human body. Therefore, we will focus our initial attention on the types and characteristics of the fluids that reside within the body. Body fluids can be classified into three types: extracellular, intracellular, and transcellular fluids. As shown in Table 3.1, nearly 60% of the body weight for an average 70 kg male is composed of these body fluids, resulting in a total fluid volume of about 40 L. The largest fraction of the fluid volume, about 36% of the body weight, consists of intracellular fluid, which is the fluid contained within the body’s cells, e.g., the fluid found within red blood cells, muscle cells, and liver cells. Extracellular fluid consists of the interstitial fluid that comprises about 17% of the body weight and the blood plasma that comprises around 4% of the body weight. Interstitial fluid circulates within the spaces interstitium( ) between cells. The interstitial fluid space represents about one-sixth of the body volume. The interstitial fluid is formed as a filtrate from the plasma within the capillaries. The capillaries are the smallest element of the cardiovascular system and represent the site where the exchange of vital substances occurs between the blood and the tissue surround- ing the capillary. -

Endothelial Glycocalyx and the Revised Starling Principle
Learners’ Corner Perspective Endothelial Glycocalyx and the Revised Starling Principle contains a steep solute concentration gradient Edward S. Crockett due to its thickness and diffusion resistance.7 To summarize, hydrostatic and oncotic pres- Pharmacology, Center for Lung Biology College of Medicine sure gradients between the microvessel lumen University of South Alabama and the interstitium are dependent on the Mobile, Alabama eGCX. United States The importance for us research scientists is not the revision itself, but the idea that the eGCX holds the power to change our understanding Introduction of a fundamental principle for which much of It is not often a textbook paradigm that has our current knowledge on edema and vascu- withstood time for 100 years is put to the test. lar health is based. Since the presence of the In 1894, E. H. Starling found discrepancies in eGCX has not been considered in other physio- data presented by Heidenhain that suggested logical studies, unexplained phenomena could “lymph was to be looked upon as a secretion be attributed to eGCX function. rather than a transudation.”1 Starling was unable to reproduce many of Heidenhain’s Brief History of eGCX discovery experimental data and realized that lymph On the path to discovering the eGCX, two filtration was not an active process of secre- developments occurred that eventually merged tion. Instead, as we learn from any physiology and led to the revised Starling Principle: textbook, it is the equilibrium between hydro- (1) The invention of the electron microscope; 2 static and oncotic pressures. However, though and (2) Continued research on fluid exchange this is not completely wrong, it is incomplete. -

Pulmonary Oedema: a Review
PULMONARY OEDEMA: A REVIEW WILLIAM H. NOBLE ABSTRACT Pulmonary oedema results from derangement of a normal physiological process which is continuously producing and removing extravascular lung water according to principles stated by Starling's equation of fluid flow across semipermeable membranes. The parameters in Starling's equation cannot all be measured. However, experiments have suggested that increased pulmonary capillary hydrostatic pressure forces fluid extravascularly, diluting the colloid osmotic pressure of tissue fluids and increasing the hydrostatic pressure of lung tissue fluids. Once these reserves and the ability of puhnonary lymphatics to remove lung water are overcome, pulmonary oedema results. This oedema can also result from reduced colloid osmotic pressure in the pulmonary capillaries and increased capillary permeability, even at low pulmonary capillary hydrostatic l~ressure. Perhaps because of the reserve created by colloid osmotic pressure of tissue fluids, hy- drostatic pressure of lung tissue fluids and lymphatic drainage, pulmonary oedema accumu- lates at a slow rate initially, and at a much more rapid rate in later stages of oedema development. The rate of oedema formation may also relate to damage to the lung created by interstitial oedema, which opens the barrier to a later stage of alveolar oedema. While lung compliance is reduced with each successive increase in oedema formation, increased shunting and hypoxaemia do not result until alveolar oedema is present, in normal lung. The diagnosis of pulmonary oedema is best made by searching for causes of oedema and by chest radiographs. The management of pulmonary oedema must begin with maintenance of oxygenation of blood. This can best be achieved by applying continuous positive pressure ventilation (CPPV). -

Perioperative Fluid Management
Ehab Farag · Andrea Kurz Editors Perioperative Fluid Management 123 Perioperative Fluid Management Ehab Farag • Andrea Kurz Editors Perioperative Fluid Management Editors Ehab Farag Andrea Kurz Professor of Anesthesiology Professor of Anesthesiology Cleveland Clinic Lerner College of Medicine Cleveland Clinic Lerner College of Medicine Director of Clinical Research Chairman of General Anesthesia Staff Anesthesiologist Cleveland Clinic General Anesthesia and Outcomes Research Cleveland Cleveland Clinic Ohio Cleveland USA Ohio USA ISBN 978-3-319-39139-7 ISBN 978-3-319-39141-0 (eBook) DOI 10.1007/978-3-319-39141-0 Library of Congress Control Number: 2016955238 © Springer International Publishing Switzerland 2016 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifi cally the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfi lms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specifi c statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. The publisher, the authors and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. Neither the publisher nor the authors or the editors give a warranty, express or implied, with respect to the material contained herein or for any errors or omissions that may have been made. -
Effective Pulmonary Capillary Pressure
Eur Respir J EDITORIAL 1988, 1, 297-301 Effective pulmonary capillary pressure F.A. Grimbert The adult respiratory distress syndrome (ARDS) is The capillary filtration rate (Qfc) is a function of the almost invariably accompanied by permeability pulmo hydrostatic (.1P) and oncotic (.17t) pressure gradients on nary oedema, usually appearing in the first few hours either side of the capillary wall, and of the capillary [1, 2]. The extent and therefore the seriousness of this filtration coefficient (Kfc) which is the product of the oedema is proportional to the filtration of fluid and exchange surface area and the coefficient of hydraulic proteins through the pulmonary vascular endothelium. conductivity. .1P is the difference between intra The extreme sensitivity of this filtration to variations of vascular and interstitial hydrostatic pressures. The capillary pressure should lead us to consider any intravascular pressure which intervenes in the filtration lowering, however small, of the latter as an essential process in West's zone 3 is the intracapiUary pressure treatment of permeability pulmonary oedema [3, 4]. [8], except perhaps in the case of microembolisation Permeability pulmonary oedema (resuhing from an [9]. As for interstitial pressure, it hardly varies, with increase in pulmonary capillary permeability), contrasts just a few exceptions (pulmonary oedema following with hydrostatic pulmonary oedema (resulting from an either a tracheal obstruction [10] or the re-expansion increase in hydrostatic pressure). The increase in cap of a collapsed lung). The variations of the term .1P illary permeability is difficult LO observe; we are there therefore depend almost wholly upon variations in fore led to define permeability pulmonary oedema as an capillary pressure. -

Fluid Physiology
Fluid Physiology Fluid Physiology: A Handbook for Anaesthesia and Critical Care Practice By Thomas Woodcock Fluid Physiology: A Handbook for Anaesthesia and Critical Care Practice By Thomas Woodcock This book first published 2019 Cambridge Scholars Publishing Lady Stephenson Library, Newcastle upon Tyne, NE6 2PA, UK British Library Cataloguing in Publication Data A catalogue record for this book is available from the British Library Copyright © 2019 by Thomas Woodcock All rights for this book reserved. No part of this book may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the copyright owner. ISBN (10): 1-5275-4031-6 ISBN (13): 978-1-5275-4031-6 CONTENTS Foreword by William W. Muir ........................................................... xii Preface ...............................................................................................xiv Chapter 1.............................................................................................. 1 Starling’s Fluid Physiology: Historic Perspective Chapter summary.................................................................................. 1 The original Starling principle ............................................................... 2 The science of Starling forces ................................................................ 3 Revising the Starling principle ............................................................... 6 Large pores -

Pulmonary Edema: a Unifying Pathophysiological Formula
ular D asc ise v as io e rd s a & C D f i o a Journal of l g a n n o r s u i s o J Tilman, J Cardiovasc Dis Diagn 2015, 3:3 ISSN: 2329-9517 Cardiovascular Diseases & Diagnosis DOI: 10.4172/2329-9517.1000194 Research Article Open Access Pulmonary Edema: A Unifying Pathophysiological Formula Vladimir Tilman* Sheba Medical Center at Tel Hashomer, Holon, Israel *Corresponding author: Vladimir Tilman, Sheba Medical Center at Tel Hashomer, Rehabilitation Hospital, Eilat 33 apt 4, Holon, 58370, Israel, Tel: 972-0547345525; E- mail: [email protected] Received date: February 9, 2015; Accepted date: March 30, 2015; Published date: April 2, 2015 Copyright: © 2015 Tilman V. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Pulmonary edema is a significant complication of a number of disease and conditions. Its main manifestation is the leakage of fluid from the pulmonary capillaries into the cavity of the alveoli through the alveolar-capillary membrane. This leads to a number of severe clinical manifestations and requires immediate treatment. The underlying mechanisms causing this complication include cardiogenic and noncardiogenic factors. A formula is proposed to attempt to unify the causes of pulmonary edema, reflecting the relationship of all factors involved in the process. The formula is simple and evident, and covers all the factors that cause or contribute to the development of pulmonary edema. It also explains the pathogenesis of cardiogenic and noncardiogenic pulmonary edema and their relationship. -

Capillary Permeability
MICROCIRCULATIONMICROCIRCULATION FUNCTIONAL ANATOMY Microcirculation is circulation of the blood through the smallest vessels of the body – arteriols, capillaries and venules. (20-50 µm) (>50 µm) (4 - 9 µm) The principal function of the microcirculation is to permit the transfer of substances (water, solutes, gases) between the vascular system and the tissues. STRUCTURE OF VESSEL WALL 1 The capillary wall is about 1 µm thick. 2 The total area of all the capillary walls in the body exceeds 500 m2. 3 The rate of blood flow in capillaries is 0.2 - 1 mm/s. 4 Transit time from arterial to venular end of a capillary is 1 - 2 seconds. ULTRASTRUCTURE OF CAPILLARY Basement membrane Endothelial cell Nucleus Vesicles 5-10 µm Fenestrationes or pores Intercellular cleft Lumen Endothelial cell MOVEMENT OF FLUID ACCROSS CAPILLARY WALL Effect of hydrostatic pressure filtration Effect of oncotic resorption pressure diffusion The diffusion, filtration and resorption of water across capillary wall occur through Intercellular clefts, pores and fenestrations. OSMOTIC PREASURE osmotic osmotic (oncotic) (oncotic) pressure pressure hydrost. pressure PRESSURE GRADIENTS ACROSS THE WALL OF CAPILLARY arteriole venule capillary Net filtration pressure: INTERSTITIAL SPACE Net filtration pressure: Pc-Pi = 36 mmHg P P c c Pc -Pi = 16 mmHg π c= 25 Net resorption pressure: Net resorption pressure: π c- π i = 25 mmHg π c- π i = 25 mmHg 37 filtration resorption 17 Pi = 1 πi ≈ 0 CAPILLARY HYDROSTATIC PRESSURE Pc = 37 - 17 mmHg INTERSTITIAL HYDROSTATIC PRESSURE Pi = 1 mmHg -
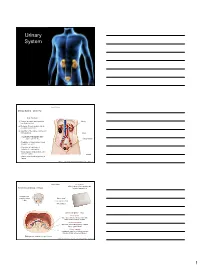
Urinary System
Urinary System Urinary System Urinary System - Overview: Major Functions: 1) Removal of organic waste products Kidney from fluids (excretion) 2) Discharge of waste products into the environment (elimination) 1 3) Regulation of the volume / [solute] / pH 3 of blood plasma Ureter HOWEVER, THE KIDNEY AIN’T JUST FOR PEE’IN… Urinary bladder • Regulation of blood volume / blood pressure (e.g., renin) • Regulation of red blood cell formation (i.e., erythropoietin) 2 • Metabolization of vitamin D to active form (Ca++ uptake) Urethra • Gluconeogenesis during prolonged fasting Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 25.1 Urinary System Renal ptosis: Kidneys drop to lower position due Functional Anatomy - Kidney: to loss of perirenal fat Located in the superior lumbar “Bar of soap” region 12 cm x 6 cm x 3 cm 150 g / kidney Layers of Supportive Tissue: Renal fascia: Peritoneal cavity Outer layer of dense fibrous connective tissue; anchors kidney in place Perirenal fat capsule: Fatty mass surrounding kidney; cushions kidney against blows Fibrous capsule: Transparent capsule on kidney; prevents infection of kidney from local tissues Kidneys are located retroperitoneal Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 25.2 1 Urinary System Functional Anatomy - Kidney: Pyelonephritis: Inflammation of the kidney Pyramids appear striped due to parallel arrangement of capillaries / collecting tubes Renal cortex Renal medulla Renal pyramids Renal papilla Renal columns Renal hilum Renal pelvis • Entrance for blood vessels