Prophylactic Nasogastric Decompression for Routine Gastrectomy Ming-Hui Pang1, Jia Xu3, Yu-Fen Wu2 and Bin Luo1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ADVANCED JOURNAL of EMERGENCY MEDICINE. in Press. Nasr Isfahani Et Al
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Advanced Journal of Emergency medicine ADVANCED JOURNAL OF EMERGENCY MEDICINE. In press. Nasr Isfahani et al Original Article DOI: 10.22114/ajem.v0i0.210 Comparison of Three Methods for NG Tube Placement in Intubated Patients in the Emergency Department Mehdi Nasr Isfahani1, Farhad Heydari1, Ahmad Azizollahi1*, Pegah Noorshargh2 1. Department of Emergency Medicine, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran. 2. Young Researchers and Elite Club, Isfahan (Khorasgan) Branch, Islamic Azad University, Isfahan, Iran. *Corresponding author: Ahmad Azizollahi; Email: [email protected] Published online: 2020-05-26 Abstract Introduction: Tubular feeding is used, in patients who cannot take food through their mouths, but their digestive system is able to digest food. This method is safe and affordable for the patient and results in maintaining the function of the digestive system and reducing the risk of infection and sepsis. Objective: The purpose of this study was to compare the three methods of the NG tube placement in intubated patients in the emergency department. Methods: This study is a randomized, prospective clinical trial conducted between 2016 and 2018. 75 patients who had been referred to the emergency department were enrolled in the study and divided into three groups, to have their NG tube insertion using either the conventional method (Group C), or using brake cable (Group B) or applying Rusch intubation stylet (Group S) for highwayman's hitch or draw hitch. Results: The mean duration of NG tube insertion was not significant between three groups (p=0.459), but the mean duration of NG tube insertion in group B was 18.43 ± 2.71 seconds and less than the other groups. -

POST OPERATIVE BOWEL MOVEMENT; Department of Surgery Unit-VI COMPARISON of PATIENTS FOLLOWING ELECTIVE STOMA CLOSURE with and Civil Hospital Karachi
POST OPERATIVE BOWEL MOVEMENT The Professional Medical Journal www.theprofesional.com ORIGINAL PROF-4174 DOI: 10.29309/TPMJ/18.4174 1. MBBS, FCPS Medical Officer, POST OPERATIVE BOWEL MOVEMENT; Department of Surgery Unit-VI COMPARISON OF PATIENTS FOLLOWING ELECTIVE STOMA CLOSURE WITH AND Civil Hospital Karachi. 2. MBBS, FCPS WITHOUT PROPHYLACTIC NASOGASTRIC TUBE IN RETURN OF POSTOPERATIVE Senior Medical Officer, BOWEL MOVEMENT Department of Surgery Unit-V Civil Hospital Karachi. 3. MBBS, FCPS 1 2 3 4 5 6 Assistant Professor Mubashir Iqbal , S. A. Sultan Ali , Khadija Tul Uzma , Farah Idrees , Adnan Aziz , Naheed Sultan Department of Surgery, ABSTRACT… Objectives: To compare early return of bowel movements in patients with DUHS. 4. MBBS, FCPS elective stoma closure with or without nasogastric tube. Place and Duration: Single surgical Assistant Professor unit, Civil Hospital, Karachi, from January 2015-August 2016. Methods: This prospective double Department of Surgery blind randomized control trial of 114 patients for elective stoma (Ileostomy, colostomy) closure DUHS. in which lottery method was used to divide the patients into control group (with nasogastric 5. MBBS, FCPS Professor tube) and study group (without nasogastric tube). Post operatively total duration from the Department of Surgery surgery till the patient passed first flatus was recorded in hours between the control and study DUHS. groups. Result: Comparison between two groups, the passage of first flatus after reversal of 6. MBBS, FCPS Professor of Surgery stoma a mean difference of 19.7 was observed in hours between the control and study groups. DUHS. Conclusion: Prophylactic nasogastric decompression in stoma closure patients can be omitted from routine postoperative period without any management problem. -

ESPEN Guideline on Home Enteral Nutrition
Clinical Nutrition 39 (2020) 5e22 Contents lists available at ScienceDirect Clinical Nutrition journal homepage: http://www.elsevier.com/locate/clnu ESPEN Guideline ESPEN guideline on home enteral nutrition * Stephan C. Bischoff a, , Peter Austin b, c, Kurt Boeykens d, Michael Chourdakis e, Cristina Cuerda f, Cora Jonkers-Schuitema g, Marek Lichota h, Ibolya Nyulasi i, Stephane M. Schneider j, Zeno Stanga k, Loris Pironi l a University of Hohenheim, Institute of Nutritional Medicine, Stuttgart, Germany b Pharmacy Department, Oxford University Hospitals NHS Foundation Trust, Oxford, UK c University College London School of Pharmacy, London, UK d AZ Nikolaas Hospital, Nutrition Support Team, Sint-Niklaas, Belgium e School of Medicine, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece f Hospital General Universitario Gregorio Maran~on, Nutrition Unit, Madrid, Spain g Amsterdam University Medical Center Location AMC, Amsterdam, the Netherlands h Intestinal Failure Patients Association “Appetite for Life”, Cracow, Poland i Department of Nutrition, Department of Rehabilitation, Nutrition and Sport, Latrobe University; Department of Medicine, Monash University, Australia j Gastroenterology and Nutrition, Centre Hospitalier Universitaire, UniversiteCote^ d’Azur, Nice, France k Division of Diabetes, Endocrinology, Nutritional Medicine and Metabolism, Bern University Hospital and University of Bern, Switzerland l Center for Chronic Intestinal Failure, St. Orsola-Malpighi University Hospital, Bologna, Italy article info summary Article history: This guideline will inform physicians, nurses, dieticians, pharmacists, caregivers and other home enteral Received 15 April 2019 nutrition (HEN) providers about the indications and contraindications for HEN, and its implementation Accepted 19 April 2019 and monitoring. Home parenteral nutrition is not included but will be addressed in a separate ESPEN guideline. -

Understanding Colic: Don't Get It Twisted
UNDERSTANDING COLIC: DON’T GET IT TWISTED Today’s Topics: What is colic? What to expect during a • Anatomy review colic visit from your • How to identify veterinarian colic • Types of colic • What to do when • Management /treatment you suspect colic • Prevention? What is colic? Abdominal Pain – a clinical sign rather than a diagnosis • Most commonly involves the gastrointestinal tract • Non GI causes of colic pain – liver, kidneys, ovaries • Other diseases that can look like colic: • Laminitis • Tying up • Pleuropneumonia • Colic is the #1 cause of early death in horses. • Incidence is around 10%, higher in stabled, working horses Abdominal Anatomy Left Side Right Side Signs of colic: • Decreased/no appetite • Decreased/ abnormal manure • Pawing • Flehmen • Stretching • Looking at sides • Lying down • Rolling • Low grade fever • Depressed MY HORSE IS COLICKING!!!! • Remove any hay/grain A word about • TPR – take your horse’s banamine: vital signs (if safe to do so) • Call your vet • Banamine – ½ dose (500lb/5ml for average size horse) Clostridial Myositis • Handwalk if anxious/painful and can keep standing • If rolling/dangerous, keep in stall and remove buckets Colic Visit: • Physical Exam • Sedation +/- banamine +/- buscopan • Palpation per rectum • Pass nasogastric tube If indicated: • Abdominal ultrasound • Abdominocentesis (belly tap) • IV fluids • Referral to hospital Colic Exam: • History: o Management changes o Recent medications o Manure production o Appetite/water intake o Deworming schedule • TPR – normal values? T: 98F-101.5F -

Incomplete Ileocecal Bypass for Ileal Pathology in Horses: 21 Cases (2012–2019)
animals Case Report Incomplete Ileocecal Bypass for Ileal Pathology in Horses: 21 Cases (2012–2019) Gessica Giusto, Anna Cerullo , Federico Labate and Marco Gandini * Department of Veterinary Sciences, University of Turin, Largo Paolo Braccini 2-5, 10095 Grugliasco (TO), Italy; [email protected] (G.G.); [email protected] (A.C.); [email protected] (F.L.) * Correspondence: [email protected] Simple Summary: The ileal pathologies represent a problem often found during colic. At exploratory laparotomy, in cases where there is involvement of the ileum and there is a suspicion of an ileocecal valve disfunction, the surgeon may be faced with the choice of whether to resect the intestinal tract involved, do nothing, or perform an ileocecal bypass without resection of the ileum. This latter tech- nique may represent a valid alternative to extensive manipulation, and it may reduce the recurrence of ileal occlusion and post-operative complication. This study aims to describe clinical findings, surgical techniques, and post-operative progress of horses who have undergone an incomplete ileocecal bypass in case of strangulating or non-strangulating ileum pathologies. Incomplete ileocecal bypass may represent an effective and safe surgical technique in these cases and in all cases of ileal pathologies treated without resection to avoid recurrence and reduce complications. Abstract: Background: Incomplete ileocecal bypass can be performed in cases in which an ileal disfunction is suspected but resection of the diseased ileum is not necessary. Objectives: To describe the clinical findings, the surgical technique, and the outcome of 21 cases of colic with ileal pathologies that underwent an incomplete ileocecal bypass. -

A Non-Invasive Real-Time Localization System for Enhanced Efficacy In
Annals of Biomedical Engineering (Ó 2015) DOI: 10.1007/s10439-015-1361-0 A Non-invasive Real-time Localization System for Enhanced Efficacy in Nasogastric Intubation 1 1 1 1 1 2 ZHENGLONG SUN, SHAOHUI FOONG , LUC MARE´CHAL, U-XUAN TAN, TEE HUI TEO, and ASIM SHABBIR 1Engineering Product Development Pillar, Singapore University of Technology and Design, 8 Somapah Road, Singapore 487372, Singapore; and 2Division of General Surgery (Upper Gastrointestinal Surgery), University Surgical Cluster, National University Health System, 5 Lower Kent Ridge Road, Singapore 119074, Singapore (Received 25 November 2014; accepted 9 June 2015) Associate Editor John H. Linehan oversaw the review of this article. Abstract—Nasogastric (NG) intubation is one of the most applications, which involves the insertion of a plastic commonly performed clinical procedures. Real-time tube through the nose, past the throat and down into localization and tracking of the NG tube passage at the larynx the stomach. Conventionally, insertion of the NG tube region into the esophagus is crucial for safety, but is lacking in current practice. In this paper, we present the design, analysis is manually performed ‘‘blind’’ without any visual aids and evaluation of a non-invasive real-time localization system or indications. It is a complex procedure and requires using passive magnetic tracking techniques to improve efficacy experience and expertise to avoid erroneous insertions9 of the clinical NG intubation process. By embedding a small as they are potentially associated with significant permanent magnet at the insertion tip of the NG tube, a morbidity and mortality.16,20 For example, if the tube wearable system containing embedded sensors around the neck can determine the absolute position of the NG tube inside the is misplaced through the larynx into the trachea and body in real-time to assist in insertion. -

Self Knotting of Nasogastric Tube: an Unusual and Rare Complication
Global Advanced Research Journal of Microbiology (ISSN: 2315-5116) Vol. 3(4) pp. 064-067, May, 2014 Available online http://garj.org/garjm/index.htm Copyright © 2014 Global Advanced Research Journals Case Report Self knotting of Nasogastric tube: an unusual and rare complication. Awe JAA (MBBS Ibadan; FWACS; FICS; DBLS; FRCS), Consultant General Surgeon and Associate Professor of Surgery, Department of Surgery, College of Health Sciences, Igbinedion University, Okada; Edo State, Nigeria. E-mail: [email protected] Accepted 07 May, 2014 Nasogastric tubes are commonly used in daily practice both for stomach decompression and for feeding purposes however the number of potential complications almost exceeds the indications for use. This innocent-looking tube can at times cause unexpected complications especially in patients with pre- existing risk factors. It has been found to have led to serious complications including respiratory distress, severe laryngeal injury, and trachea-esophageal puncture. Knotting of small-bore feeding tubes and nasogastric tubes during insertion and removal is rare; knotting of large-caliber nasogastric tubes is even more uncommon. However most of this morbidity is avoidable with careful attention to detail when placing the tube and careful management of the tube on a day to day basis. A knot occurring in the distal end of a nasogastric tube is a rare complication. This is either recognized once the tube is completely removed, or if resistance is encountered during its removal, when the knot presses upon the posterior aspect of the nasal area. We hereby report a knotted nasogastric tube on one of our long stay patient in our health facility inserted for feeding and administration of medication. -

Early Removal Versus Conventional Removal of Nasogastric Tube After Abdominal Surgery: a Prospective Randomized Controlled Study
International Surgery Journal Goudar BV et al. Int Surg J. 2017 Jan;4(1):229-232 http://www.ijsurgery.com pISSN 2349-3305 | eISSN 2349-2902 DOI: http://dx.doi.org/10.18203/2349-2902.isj20164424 Original Research Article Early removal versus conventional removal of nasogastric tube after abdominal surgery: a prospective randomized controlled study Bhimanagouda Venkanagouda Goudar*, Eshwar B. Kalburgi, Hanumaraddi L. Giraddi, Mohammedgouse Abdulsattar Karikazi Department of Surgery, S. N. Medical College and HSK, Research Centre, Bagalkot, Karnataka, India Received: 23 November 2016 Accepted: 05 December 2016 *Correspondence: Dr. Bhimanagouda Venkanagouda Goudar, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Post-operative nasogastric decompression was introduced by Levin’s in 1926 and is intended to drain secretions and gas from the upper gastro-intestinal tract, thereby reducing vomiting, abdominal distension, abdominal discomfort, to prevent anastamotic leak and wound dehiscence. Methods: The current study was carried among all patients of abdominal surgeries that were conducted in S.N. Medical College and H.S.K. Hospital and Research Centre, Bagalkot, Karnataka, India. Among the total sample of 100 cases, patients undergoing abdominal surgeries nasogastric output, absence of emesis and no increasing abdominal discomfort were randomized into two groups. Results: In the current study majority of the patients (79%) were in the age group of 21 - 60 years and male to female ratio is 2.3:1. -

Veterinary Practitioners' Selection of Diagnostic Tests for the Primary Evaluation of Colic in the Horse L
Downloaded from http://vetrecordopen.bmj.com/ on November 10, 2015 - Published by group.bmj.com Horses and other equids Veterinary practitioners’ selection of diagnostic tests for the primary evaluation of colic in the horse L. Curtis, I. Trewin, G. C. W. England, J. H. Burford, S. L. Freeman To cite: Curtis L, et al. ABSTRACT abdominal pain, but other conditions such as ’ Veterinary practitioners The aim of this study was to survey veterinary thoracic pain can have similarities in presenta- selection of diagnostic tests practitioners’ selection of diagnostic tests for horses tion (false colic). An early and accurate diag- for the primary evaluation of with clinical signs of abdominal pain. A questionnaire colic in the horse. Vet Rec nosis is particularly important for critical cases, was distributed to veterinary surgeons involved in the Open 2015;2:e000145. where the degree, duration and severity of doi:10.1136/vetreco-2015- primary evaluation of horses with abdominal pain, ’ pathology will impact upon outcome. 000145 including the respondent s demographics, selection of diagnostic tests and factors affecting decision-making. Many diagnostic tests can be used to evalu- Data analysis included descriptive analysis, ate horses with abdominal pain, and these ▸ Prepublication history and categorisation of free text and simple univariable vary in their cost and the facilities and level additional material is of expertise required to perform the techni- available. To view please visit correlations to explore the relationships between the journal online independent variables and the relative self-estimated ques and interpreting outcomes. The (http://dx.doi.org/10.1136/ frequency that diagnostic tests were performed. -

Esophageal Perforation Following Orogastric Suction Catheter Insertion in an Elderly Patient
ESOPHAGEAL PERFORATION FOLLOWING OROGASTRIC SUCTION CATHETER INSERTION IN AN ELDERLY PATIENT ROLAND N. KADDOUM*, FADI FARAH**, RITA W. SAROUFIM*** AND SALAH M. ZEINELDINE**** Abstract Esophageal rupture has been described following iatrogenic manipulation. In this report, we present an elderly lady admitted to the operative theater for laparoscopic cholecystectomy. Multiple intra-operative attempts to place a flexible orogastric tube were unsuccessful because of failure to advance. Post-operatively, the patient developed sepsis and a right pleural effusion. She was transferred to the Intensive Care Unit and she was treated with antibiotics. Radiologic evaluation confirmed an esophago-pleural fistula. Surgical repair was urgently performed for closure of fistula and lung decortication. The patient recovered and was discharged home. Introduction Esophageal perforation is a serious complication of procedures performed on or around the esophagus and is mainly iatrogenic1,2. Esophageal perforation is most frequently encountered during endoscopic procedures3 but also during nasogastric tube placement4-9, endotracheal intubation10-14, stricture dilation15,16 and during the use of oesophageal bougies17. This complication has a poor prognosis with inadequate management3. While providing anesthesia to surgical patients, anesthesists frequently insert esophageal dilators, nasogastric tubes or orogastric tubes/suction catheters. Such interventions may cause trauma to soft tissue structures. We describe a case of esophageal perforation secondary to the orogastric insertion of a simple 14 french suction catheter used routinely to deflate the stomach during laparoscopic cholecystectomy. To our knowledge, this is the first case report describing esophageal perforation following the insertion of a flexible orogastric 14Fr suction catheter in an adult patient undergoing laparoscopic cholecystectomy. * MD, Assistant Professor of Anesthesiology, American University of Beirut Medical Center, Department of Anesthesiology, Beirut, Lebanon (Riad Solh 1107-2020). -

Jejunoileal Anastomosis Technique in Six Horses
Reprinted in the IVIS website with the permission of AAEP Close window to return to IVIS SURGERY I Jejunoileal Anastomosis Technique in Six Horses Dawn A. Loesch, DVM; Dwayne H. Rodgerson, DVM, MS, Diplomate ACVS; Gregory R. Haines, DVM; and Bruce C. Watt, DVM, Diplomate ACVS A two-layer, hand-sewn, end-to-end jejunoileal anastomosis technique can result in few postoperative complications. Advantages to this technique include maintaining the normal ileocecal valve, shorter surgical time, and a generally uncomplicated postoperative recovery in the majority of horses. Au- thor’s address: Department of Large Animal Clinical Sciences, College of Veterinary Medicine, University of Florida, Gainesville, FL 32610-0136. © 2001 AAEP. 1. Introduction to-end anastomosis of the jejunum to the ileum with Strangulating small intestinal lesions represent a a simple continuous mucosal layer followed by a significant cause of equine colic requiring surgical simple continuous seromuscular layer has not been correction. Various methods of anastomosis follow- reported in the literature. The purpose of this re- ing resection of affected bowel have been described, port is to describe six horses undergoing hand-sewn, depending on the portion of bowel affected by the end-to-end jejunoileal anastomosis in two simple primary lesion. When any portion of the ileum is continuous layers following resection of strangu- resected following involvement in a strangulating lated small intestine and to evaluate the short-term lesion, creation of a jejunocecostomy has been -
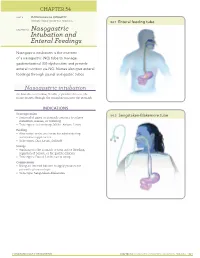
Intubation and Enteral Feedings
Online Images: Enteral Feeding Tube, Sengstaken-Blakemore Tube CHAPTER 54 UNIT 4 PHYSIOLOGICAL INTEGRITY SECTION: REDUCTION OF RISK POTENTIAL 54.1 Enteral feeding tube CHAPTER 54 Nasogastric Intubation and Enteral Feedings Nasogastric intubation is the insertion of a nasogastric (NG) tube to manage gastrointestinal (GI) dysfunction and provide enteral nutrition via NG. Nurses also give enteral feedings through jejunal and gastric tubes. Nasogastric intubation An NG tube is a hollow, flexible, cylindrical device the nurse inserts through the nasopharynx into the stomach. INDICATIONS Decompression 54.2 Sengstaken-Blakemore tube ● Removal of gases or stomach contents to relieve distention, nausea, or vomiting ● Tube types: Salem sump, Miller‑Abbott, Levin Feeding ● Alternative to the oral route for administering nutritional supplements ● Tube types: Duo, Levin, Dobhoff Lavage ● Washing out the stomach to treat active bleeding, ingestion of poison, or for gastric dilation ● Tube types: Ewald, Levin, Salem sump Compression ● Using an internal balloon to apply pressure for preventing hemorrhage ● Tube type: Sengstaken‑Blakemore FUNDAMENTALS FOR NURSING CHAPTER 54 NASOGASTRIC INTUBATION AND ENTERAL FEEDINGS 321 CONSIDERATIONS ● After placement verification, secure the NG tube on the nose, avoiding pressure on the nares. PREPROCEDURE ◯ Confirm placement with an x‑ray. ◯ Injecting air into the tube and then listening over the NURSING ACTIONS abdomen is not an acceptable practice. ● Review the prescription and purpose, plan for drainage ● If the tube is not in the stomach, advance it 5 cm (2 in), or suction, and understand the need for placement for and repeat the placement check. diagnostic purposes. ● Clamp the NG tube, or connect it to the suction device.