Gao-21-387, Covid-19
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Tea Party: a Party Within a Party a Dissertation Submitted to The
The Tea Party: A Party Within a Party A Dissertation submitted to the Faculty of the Graduate School of Arts and Sciences of Georgetown University in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Government By Rachel Marie Blum, M.A. Washington, DC March 22, 2016 Copyright c 2016 by Rachel Marie Blum All Rights Reserved ii The Tea Party: A Party Within a Party Rachel Marie Blum, M.A. Dissertation Advisor: Hans Noel, Ph.D. Abstract It is little surprise that conservatives were politically disaffected in early 2009, or that highly conservative individuals mobilized as a political movement to protest ‘big government’ and Obama’s election. Rather than merely directing its animus against liberals, the Tea Party mobilized against the Republican Party in primaries and beyond. This dissertation draws from original survey, interview, Tea Party blog, and social network datasets to explain the Tea Party’s strategy for mobilization as a ‘Party within a Party’. Integrating new data on the Tea Party with existing theories of political parties, I show that the Tea Party’s strategy transcends the focused aims of a party faction. Instead, it works to co-opt the Republican Party’s political and electoral machinery in order to gain control of the party. This dissertation offers new insights on the Tea Party while developing a theory of intra-party mobilization that endures beyond the Tea Party. Index words: Dissertations, Government, Political Science, Political Parties, Tea Party iii Dedication To M.L.B., and all others who are stronger than they know. -

Upcoming BISM Events
Upcoming BISM Events Date Dept./Location Event 1/1 All Locations Closed for New Year’s Day Holiday 1/8 Rehabilitation-Hagerstown Seniors: Support Group Meeting at Church 1/8 Rehabilitation-Baltimore Seniors: Blind Vets Workshop at VA Baltimore 1/8 Annapolis Maryland General Assembly Session Begins 1/9 Aberdeen/Edgewood BSC GPC AbilityOne BSC Program Training 1/10 Rehabilitation-Baltimore Seniors: Support Group Meeting 1/14 JB Andrews BSC GPC AbilityOne BSC Program Training 1/15 Ft. Knox BSC GPC AbilityOne BSC Program Training 1/15 Rehabilitation-Baltimore Seniors: Outing to NFB Independence Market 1/20 All Locations Closed for Martin Luther King Jr. Holiday 1/21 Dover BSC GPC AbilityOne BSC Program Training 1/22 Rehabilitation-Hagerstown Seniors: SAIL Outing at Valley Mall 1/23 Rehabilitation-Salisbury Seniors: SAIL Bowling Outing 1/24 Rehabilitation-Baltimore Seniors: Blakehurst Blindness 101 Skills Workshop at BISM 1/27 Rehabilitation-Salisbury Seniors: VIPS Support Group Meeting 1/30 Baltimore Book Club Meeting - 4:00 pm BISM 2020 Holiday Schedule BISM Locations AbilityOne Base Supply Centers New Year’s Day—Wednesday, January 1 New Year’s Day—Wednesday, January 1 Martin Luther King Jr. Day—Monday, January 20 Martin Luther King Jr. Day—Monday, January 20 Presidents’ Day—Monday, February 17 Presidents’ Day—Monday, February 17 Memorial Day—Monday, May 25 Memorial Day—Monday, May 25 Independence Day—Friday, July 3 Independence Day—Friday, July 3 Labor Day—Monday, September 7 Labor Day—Monday, September 7 Veterans Day—Wednesday, November 11 Columbus Day—Monday, October 12 Thanksgiving—Thursday, November 26 Veterans Day—Wednesday, November 11 Day after Thanksgiving—Friday, November 27 Thanksgiving—Thursday, November 26 Christmas—Friday, December 25 Christmas—Friday, December 25 2020 Shutdown Guidelines for BISM Locations (BSCs excluded): Independence Day shutdown - BISM will close at the end of the business day on Wednesday, July 1. -
2021 License Renewal Information for Physician Assistants
2021 LICENSE RENEWAL INFORMATION FOR PHYSICIAN ASSISTANTS For inquiries, please call 410-764-4705 or 1-800-492-6836 ext. 4705, Monday through Friday from 8:00 am to 4:30 pm. License renewal will begin on May 3, 2021. In order to maintain an active Maryland license, licensees are required to submit a renewal application and renewal fee of $160.00* to the Board by June 30, 2021. The online renewal application is accessible 24 hours a day, 7 days a week on the Board’s website starting May 3, 2021 through June 30, 2021. The online system will not be available after June 30, 2021. Failure to renew by 11:59 p.m. (EST) on June 30, 2021, will result in expiration of your license to practice in Maryland. Licensees who fail to renew by the expiration date must apply for reinstatement of their license and submit a new criminal history records check. If you do not plan to renew your license, no action is required from you. To login and complete the renewal application, you will need: • Your Maryland license number; • Last 4 digits of your social security number; and • A Visa, MasterCard or Discover credit card only. The Board no longer accepts payment of license renewal fees by check or money order. You cannot access the online application if: • Your license is expired. If your license has expired, you must reinstate it by completing and submitting a reinstatement application. Click the following link for a reinstatement application: https://www.mbp.state.md.us/forms/parein.pdf • You have an unresolved tax liability issue with the Comptroller’s Office of Maryland. -

Congressional Documents: Browse.” GPO Access
US CONGRESSIONAL PUBLICATIONS BRIDGEPORT PUBLIC LIBRARY ONLINE AND HARDCOPY DOCUMENTS AS OF DEC. 28, 2005 Stephen Kwasnik Federal Documents Librarian Online and in Hardcopy at the Bridgeport Public Library Part 1: Congressional Reports and Documents: The US Serial Set Part 2: Public Laws and the U.S. Code Part 3: Congressional Bills Part 4: The Congressional Record/Congressional Debate Part 5: Senate Treaties Part 6: Congressional Hearings PART 1: HOUSE AND SENATE DOCUMENTS AND REPORTS (Also House and Senate Joint Resolutions, Committee Reports, Joint Conference Reports.) “The Serial Set does not normally include the text of Congressional Debates, Bills, Resolutions, Hearings, Committee Prints and Publications from support agencies of Congress. [but] by special order some selected hearings and bill texts are included, especially in the 19th Century.” Finding Aids: A. Online Finding Aids 1. Schedule of Serial Set Volumes Time Coverage: 1970 / 91st Congress -Present McKinney, Richard J. “Schedule of Volumes to the U.S. Congressional Serial Set 1970 to Current.” LLSDC’S Legislative Sourcebook. 2005. Law Librarians Society of Washington, D.C. 25 Sept. 2005 http://www.llsdc.org/sourcebook/sch-v.htm This information is also found on the GPO Access website at http://www.gpoaccess.gov/serialset/numerical.html 2. Numerical Lists of Documents and Reports (Online) (See Illustration 2) Time Coverage: 1991-2002 United States. United States Government Printing Office. “U.S. Congressional Serial Set: Numerical Lists of Documents and Reports. “ GPO Access. -

The Forgotten Encyclopedia
Linn Holmberg Linn Holmberg In mid-eighteenth-century Paris, two young monks from the Benedictine Congregation of Saint-Maur – also known as the Maurists – started compil- ing a universal dictionary of arts, crafts, and sciences. The work was initiated simultaneously with what would become one of the most famous literary enterprises in Western intellectual history: the Encyclopédie of Diderot and d’Alembert. While the dictionary of the philosophes eventually turned into Encyclopedia Forgotten The a controversial but successful best-seller, considered as the most important medium of Enlightenment thought, the Benedictines never finished or pub- lished their work. After almost a decade, the manuscripts were put aside in the monastery library, and were soon forgotten. This dissertation explores the history and contents of the Maurists’ enter- prise. The project is situated within its monastic environment of production, the history of the encyclopedic dictionary, and the Enlightenment culture. The study shows that the Maurists early found themselves in a rival situation with the embryonic Encyclopédie, and that the two projects had several com- mon denominators that distinguished them from the predecessors within the genre. At the same time, the Maurists were making a dictionary unique in the eighteenth century. The study provides new perspectives on the Encyclopédie of Diderot and d’Alembert, the intellectual activities of the Congregation of Saint-Maur, as well as the editor in charge of the Maurist dictionary: Dom Antoine-Joseph Pernety, -
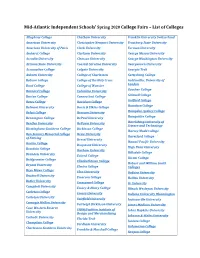
Mid-Atlantic Independent Schools' Spring 2020 College Fairs – List Of
Mid-Atlantic Independent Schools’ Spring 2020 College Fairs – List of Colleges Allegheny College Chatham University Franklin University Switzerland American University Christopher Newport University Frostburg State University American University of Paris Clark University Furman University Amherst College Clarkson University George Mason University Arcadia University Clemson University George Washington University Arizona State University Coastal Carolina University Georgetown University Assumption College Colgate University Georgia Tech Auburn University College of Charleston Gettysburg College Babson College College of the Holy Cross Goldsmiths, University of London Bard College College of Wooster Goucher College Barnard College Columbia University Grinnell College Barton College Connecticut College Guilford College Bates College Davidson College Hamilton College Belmont University Davis & Elkins College Hampden-Sydney College Beloit College Denison University Hampshire College Bennington College DePaul University Harrisburg University of Bentley University DePauw University Science and Technology Birmingham-Southern College Dickinson College Harvey Mudd College Bon Secours Memorial College Drew University Haverford College of Nursing Drexel University Hawaii Pacific University Boston College Duquesne University High Point University Bowdoin College Durham University Hillsdale College Brandeis University Eckerd College Hiram College Bridgewater College Elizabethtown College Hobart and William Smith Bryant University Elmira College Colleges