Catenin/T-Cell Factor Signaling Identified by Gene Expression Profiling of Ovarian Endometrioid Adenocarcinomas1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Landscape of Genomic Imprinting Across Diverse Adult Human Tissues
Downloaded from genome.cshlp.org on October 3, 2021 - Published by Cold Spring Harbor Laboratory Press Research The landscape of genomic imprinting across diverse adult human tissues Yael Baran,1 Meena Subramaniam,2 Anne Biton,2 Taru Tukiainen,3,4 Emily K. Tsang,5,6 Manuel A. Rivas,7 Matti Pirinen,8 Maria Gutierrez-Arcelus,9 Kevin S. Smith,5,10 Kim R. Kukurba,5,10 Rui Zhang,10 Celeste Eng,2 Dara G. Torgerson,2 Cydney Urbanek,11 the GTEx Consortium, Jin Billy Li,10 Jose R. Rodriguez-Santana,12 Esteban G. Burchard,2,13 Max A. Seibold,11,14,15 Daniel G. MacArthur,3,4,16 Stephen B. Montgomery,5,10 Noah A. Zaitlen,2,19 and Tuuli Lappalainen17,18,19 1The Blavatnik School of Computer Science, Tel-Aviv University, Tel Aviv 69978, Israel; 2Department of Medicine, University of California San Francisco, San Francisco, California 94158, USA; 3Analytic and Translational Genetics Unit, Massachusetts General Hospital, Boston, Massachusetts 02114, USA; 4Program in Medical and Population Genetics, Broad Institute of Harvard and MIT, Cambridge, Massachusetts 02142, USA; 5Department of Pathology, Stanford University, Stanford, California 94305, USA; 6Biomedical Informatics Program, Stanford University, Stanford, California 94305, USA; 7Wellcome Trust Center for Human Genetics, Nuffield Department of Clinical Medicine, University of Oxford, Oxford, OX3 7BN, United Kingdom; 8Institute for Molecular Medicine Finland, University of Helsinki, 00014 Helsinki, Finland; 9Department of Genetic Medicine and Development, University of Geneva, 1211 Geneva, Switzerland; -

Análise Integrativa De Perfis Transcricionais De Pacientes Com
UNIVERSIDADE DE SÃO PAULO FACULDADE DE MEDICINA DE RIBEIRÃO PRETO PROGRAMA DE PÓS-GRADUAÇÃO EM GENÉTICA ADRIANE FEIJÓ EVANGELISTA Análise integrativa de perfis transcricionais de pacientes com diabetes mellitus tipo 1, tipo 2 e gestacional, comparando-os com manifestações demográficas, clínicas, laboratoriais, fisiopatológicas e terapêuticas Ribeirão Preto – 2012 ADRIANE FEIJÓ EVANGELISTA Análise integrativa de perfis transcricionais de pacientes com diabetes mellitus tipo 1, tipo 2 e gestacional, comparando-os com manifestações demográficas, clínicas, laboratoriais, fisiopatológicas e terapêuticas Tese apresentada à Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo para obtenção do título de Doutor em Ciências. Área de Concentração: Genética Orientador: Prof. Dr. Eduardo Antonio Donadi Co-orientador: Prof. Dr. Geraldo A. S. Passos Ribeirão Preto – 2012 AUTORIZO A REPRODUÇÃO E DIVULGAÇÃO TOTAL OU PARCIAL DESTE TRABALHO, POR QUALQUER MEIO CONVENCIONAL OU ELETRÔNICO, PARA FINS DE ESTUDO E PESQUISA, DESDE QUE CITADA A FONTE. FICHA CATALOGRÁFICA Evangelista, Adriane Feijó Análise integrativa de perfis transcricionais de pacientes com diabetes mellitus tipo 1, tipo 2 e gestacional, comparando-os com manifestações demográficas, clínicas, laboratoriais, fisiopatológicas e terapêuticas. Ribeirão Preto, 2012 192p. Tese de Doutorado apresentada à Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo. Área de Concentração: Genética. Orientador: Donadi, Eduardo Antonio Co-orientador: Passos, Geraldo A. 1. Expressão gênica – microarrays 2. Análise bioinformática por module maps 3. Diabetes mellitus tipo 1 4. Diabetes mellitus tipo 2 5. Diabetes mellitus gestacional FOLHA DE APROVAÇÃO ADRIANE FEIJÓ EVANGELISTA Análise integrativa de perfis transcricionais de pacientes com diabetes mellitus tipo 1, tipo 2 e gestacional, comparando-os com manifestações demográficas, clínicas, laboratoriais, fisiopatológicas e terapêuticas. -

Noelia Díaz Blanco
Effects of environmental factors on the gonadal transcriptome of European sea bass (Dicentrarchus labrax), juvenile growth and sex ratios Noelia Díaz Blanco Ph.D. thesis 2014 Submitted in partial fulfillment of the requirements for the Ph.D. degree from the Universitat Pompeu Fabra (UPF). This work has been carried out at the Group of Biology of Reproduction (GBR), at the Department of Renewable Marine Resources of the Institute of Marine Sciences (ICM-CSIC). Thesis supervisor: Dr. Francesc Piferrer Professor d’Investigació Institut de Ciències del Mar (ICM-CSIC) i ii A mis padres A Xavi iii iv Acknowledgements This thesis has been made possible by the support of many people who in one way or another, many times unknowingly, gave me the strength to overcome this "long and winding road". First of all, I would like to thank my supervisor, Dr. Francesc Piferrer, for his patience, guidance and wise advice throughout all this Ph.D. experience. But above all, for the trust he placed on me almost seven years ago when he offered me the opportunity to be part of his team. Thanks also for teaching me how to question always everything, for sharing with me your enthusiasm for science and for giving me the opportunity of learning from you by participating in many projects, collaborations and scientific meetings. I am also thankful to my colleagues (former and present Group of Biology of Reproduction members) for your support and encouragement throughout this journey. To the “exGBRs”, thanks for helping me with my first steps into this world. Working as an undergrad with you Dr. -

Communication in Asthmatic Airways Inflammation and Intercellular Gene
Gene Expression Patterns of Th2 Inflammation and Intercellular Communication in Asthmatic Airways This information is current as David F. Choy, Barmak Modrek, Alexander R. Abbas, Sarah of September 28, 2021. Kummerfeld, Hilary F. Clark, Lawren C. Wu, Grazyna Fedorowicz, Zora Modrusan, John V. Fahy, Prescott G. Woodruff and Joseph R. Arron J Immunol 2011; 186:1861-1869; Prepublished online 27 December 2010; Downloaded from doi: 10.4049/jimmunol.1002568 http://www.jimmunol.org/content/186/3/1861 Supplementary http://www.jimmunol.org/content/suppl/2010/12/27/jimmunol.100256 http://www.jimmunol.org/ Material 8.DC1 References This article cites 63 articles, 10 of which you can access for free at: http://www.jimmunol.org/content/186/3/1861.full#ref-list-1 Why The JI? Submit online. by guest on September 28, 2021 • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2011 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Gene Expression Patterns of Th2 Inflammation and Intercellular Communication in Asthmatic Airways David F. -

Chip-Seq Analysis to Explore DNA Replication Profile in Trifluridine
ANTICANCER RESEARCH 39 : 3565-3570 (2019) doi:10.21873/anticanres.13502 ChIP-seq Analysis to Explore DNA Replication Profile in Trifluridine-treated Human Colorectal Cancer Cells In Vitro TAKASHI KOBUNAI 1* , KAZUAKI MATSUOKA 2* and TEIJI TAKECHI 1 1Taiho Pharmaceutical Co., Ltd., Translational Research Laboratory, Tokyo, Japan; 2Taiho Pharmaceutical Co., Ltd., Translational Research Laboratory (Tokushima office), Tokushima, Japan Abstract. Background/Aim: Trifluridine (FTD) is a key efficacy and a tolerable safety profile for previously treated component of the novel oral antitumor drug trifluridine/ metastatic colorectal cancer (2). tipiracil that has been approved for the treatment of metastatic In basic research, FTD, the active antitumor component of colorectal cancer. In this study, a comprehensive analysis of FTD/TPI, is an antineoplastic thymidine analog (3), efficiently DNA replication profile in FTD-treated colon cancer cells was incorporated into genomic DNA in tumor cells (4-7). FTD performed. Materials and Methods: HCT-116 cells were transported into the cytoplasm of tumor cells by equilibrative exposed to BrdU or FTD and subjected to DNA immuno- nucleoside transporter 1 and 2 is phosphorylated to mono- precipitation. Immunoprecipitated DNA was sequenced; the phosphate, diphosphate, and triphosphate (FTD-TP) forms by density of aligned reads along the genome was calculated. Peak thymidine kinase 1 and nucleoside diphosphate kinase, finding, gene ontology, and motif analysis were performed using respectively. DNA polymerase α incorporates FTD-TP into MACS, GREAT, and MEME, respectively. Results: We identified DNA at sites aligned with adenine on the opposite strand (8). 6,043 and 5,080 high-confidence FTD and BrdU peaks in HCT- FTD/TPI exerts its antitumor activity in vivo predominantly 116 cells, respectively. -

Chromosome 20 Shows Linkage with DSM-IV Nicotine Dependence in Finnish Adult Smokers
Nicotine & Tobacco Research, Volume 14, Number 2 (February 2012) 153–160 Original Investigation Chromosome 20 Shows Linkage With DSM-IV Nicotine Dependence in Finnish Adult Smokers Kaisu Keskitalo-Vuokko, Ph.D.,1 Jenni Hällfors, M.Sc.,1,2 Ulla Broms, Ph.D.,1,3 Michele L. Pergadia, Ph.D.,4 Scott F. Saccone, Ph.D.,4 Anu Loukola, Ph.D.,1,3 Pamela A. F. Madden, Ph.D.,4 & Jaakko Kaprio, M.D., Ph.D.1,2,3 1 Hjelt Institute, Department of Public Health, University of Helsinki, Helsinki, Finland 2 Institute for Molecular Medicine Finland (FIMM), University of Helsinki, Helsinki, Finland 3 National Institute for Health and Welfare (THL), Helsinki, Finland 4 Department of Psychiatry, Washington University School of Medicine, St. Louis, MO Corresponding Author: Jaakko Kaprio, M.D., Ph.D., Department of Public Health, University of Helsinki, PO Box 41 (Manner- heimintie 172), Helsinki 00014, Finland. Telephone: +358-9-191-27595; Fax: +358-9-19127570; E-mail: [email protected] Received February 5, 2011; accepted June 21, 2011 2009). Despite several gene-mapping studies, the genes underlying Abstract liability to nicotine dependence (ND) remain largely unknown. Introduction: Chromosome 20 has previously been associated Recently, Han, Gelernter, Luo, and Yang (2010) performed a with nicotine dependence (ND) and smoking cessation. Our meta-analysis of 15 genome-wide linkage scans of smoking aim was to replicate and extend these findings. behavior. Linkage signals were observed on chromosomal regions 17q24.3–q25.3, 5q33.1–q35.2, 20q13.12–32, and 22q12.3–13.32. Methods: First, a total of 759 subjects belonging to 206 Finnish The relevance of the chromosome 20 finding is highlighted families were genotyped with 18 microsatellite markers residing by the fact that CHRNA4 encoding the nicotinic acetylcholine on chromosome 20, in order to replicate previous linkage findings. -

Functional Specialization of Human Salivary Glands and Origins of Proteins Intrinsic to Human Saliva
UCSF UC San Francisco Previously Published Works Title Functional Specialization of Human Salivary Glands and Origins of Proteins Intrinsic to Human Saliva. Permalink https://escholarship.org/uc/item/95h5g8mq Journal Cell reports, 33(7) ISSN 2211-1247 Authors Saitou, Marie Gaylord, Eliza A Xu, Erica et al. Publication Date 2020-11-01 DOI 10.1016/j.celrep.2020.108402 Peer reviewed eScholarship.org Powered by the California Digital Library University of California HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Cell Rep Manuscript Author . Author manuscript; Manuscript Author available in PMC 2020 November 30. Published in final edited form as: Cell Rep. 2020 November 17; 33(7): 108402. doi:10.1016/j.celrep.2020.108402. Functional Specialization of Human Salivary Glands and Origins of Proteins Intrinsic to Human Saliva Marie Saitou1,2,3, Eliza A. Gaylord4, Erica Xu1,7, Alison J. May4, Lubov Neznanova5, Sara Nathan4, Anissa Grawe4, Jolie Chang6, William Ryan6, Stefan Ruhl5,*, Sarah M. Knox4,*, Omer Gokcumen1,8,* 1Department of Biological Sciences, University at Buffalo, The State University of New York, Buffalo, NY, U.S.A 2Section of Genetic Medicine, Department of Medicine, University of Chicago, Chicago, IL, U.S.A 3Faculty of Biosciences, Norwegian University of Life Sciences, Ås, Viken, Norway 4Program in Craniofacial Biology, Department of Cell and Tissue Biology, School of Dentistry, University of California, San Francisco, CA, U.S.A 5Department of Oral Biology, School of Dental Medicine, University at Buffalo, The State University of New York, Buffalo, NY, U.S.A 6Department of Otolaryngology, School of Medicine, University of California, San Francisco, CA, U.S.A 7Present address: Weill-Cornell Medical College, Physiology and Biophysics Department 8Lead Contact SUMMARY Salivary proteins are essential for maintaining health in the oral cavity and proximal digestive tract, and they serve as potential diagnostic markers for monitoring human health and disease. -

Development and Validation of a Serum Biomarker Panel for The
Journal of Cancer 2017, Vol. 8 2346 Ivyspring International Publisher Journal of Cancer 2017; 8(12): 2346-2355. doi: 10.7150/jca.19465 Research Paper Development and Validation of a Serum Biomarker Panel for the Detection of Esophageal Squamous Cell Carcinoma through RNA Transcriptome Sequencing Shan Xing1,2†, Xin Zheng1,2†, Li-Qiang Wei4†, Shi-Jian Song5, Dan Liu1,3, Ning Xue1,3, Xiao-Min Liu1,2, Mian-Tao Wu1,2, Qian Zhong1,3, Chu-Mei Huang6, Mu-Sheng Zeng1,3, Wan-Li Liu1,2 1. State Key Laboratory of Oncology in Southern China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China 2. Department of Clinical Laboratory, Sun Yat-sen University Cancer Center, Guangzhou, China 3. Department of Experimental Research, Sun Yat-sen University cancer center, Guangzhou, China 4. Department of Clinical Laboratory, Shaanxi Provincial People’s Hospital, Xian, China 5. Guangdong Experimental High School, Guangzhou, China 6. Department of Laboratory Medicine, Sun Yat-sen University First Affiliated Hospital, Guangzhou, China † Xing S, Zheng X and Wei LQ contributed equally to this work Corresponding authors: Wan-Li Liu. Email: [email protected]; Phone: 86-020-87343196. Department of Clinical Laboratory, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East,Guangzhou 510060, P.R. China. Mu-Sheng Zeng; Email: [email protected] Phone: +86-020-87343192. Department of Experimental Research, Sun Yat-sen University cancer center, 651 Dongfeng Road East,Guangzhou 510060, P.R.China © Ivyspring International Publisher. This is an open access article distributed under the terms of the Creative Commons Attribution (CC BY-NC) license (https://creativecommons.org/licenses/by-nc/4.0/). -
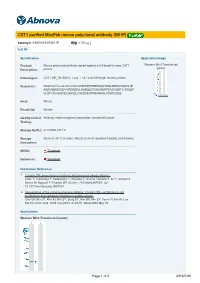
CST1 Purified Maxpab Mouse Polyclonal Antibody (B01P)
CST1 purified MaxPab mouse polyclonal antibody (B01P) Catalog # : H00001469-B01P 規格 : [ 50 ug ] List All Specification Application Image Product Mouse polyclonal antibody raised against a full-length human CST1 Western Blot (Transfected lysate) Description: protein. Immunogen: CST1 (NP_001889.2, 1 a.a. ~ 141 a.a) full-length human protein. Sequence: MAQYLSTLLLLLATLAVALAWSPKEEDRIIPGGIYNADLNDEWVQRALHF AISEYNKATKDDYYRRPLRVLRARQQTVGGVNYFFDVEVGRTICTKSQP NLDTCAFHEQPELQKKQLCSFEIYEVPWENRRSLVKSRCQES enlarge Host: Mouse Reactivity: Human Quality Control Antibody reactive against mammalian transfected lysate. Testing: Storage Buffer: In 1x PBS, pH 7.4 Storage Store at -20°C or lower. Aliquot to avoid repeated freezing and thawing. Instruction: MSDS: Download Datasheet: Download Publication Reference 1. Cystatin SN Upregulation in Patients with Seasonal Allergic Rhinitis. Imoto Y, Tokunaga T, Matsumoto Y, Hamada Y, Ono M, Yamada T, Ito Y, Arinami T, Okano M, Noguchi E, Fujieda SPLoS One. 2013;8(8):e67057. doi: 10.1371/journal.pone.0067057. 2. Upregulation of the cysteine protease inhibitor, Cystatin SN, contributes to cell proliferation and cathepsin inhibition in gastric cancer. Choi EH, Kim JT, Kim JH, Kim SY, Song EY, Kim JW, Kim SY, Yeom YI, Kim IH, Lee HG.Clin Chim Acta. 2009 Aug;406(1-2):45-51. Epub 2009 May 19. Applications Western Blot (Transfected lysate) Page 1 of 2 2016/5/20 Western Blot analysis of CST1 expression in transfected 293T cell line (H00001469-T01) by CST1 MaxPab polyclonal antibody. Lane 1: CST1 transfected lysate(15.51 KDa). Lane 2: Non-transfected lysate. Protocol Download Gene Information Entrez GeneID: 1469 GeneBank NM_001898.2 Accession#: Protein NP_001889.2 Accession#: Gene Name: CST1 Gene Alias: - Gene cystatin SN Description: Omim ID: 123855 Gene Ontology: Hyperlink Gene Summary: The cystatin superfamily encompasses proteins that contain multiple cystatin-like sequences. -

A Comparative Genomics Approach to Using High-Throughput
The University of Maine DigitalCommons@UMaine Honors College 5-2013 A Comparative Genomics Approach to Using High-Throughput Gene Expression Data to Study Limb Regeneration in Ambystoma Mexicanum and Danio Rerio: Developing a More Completely Annotated Database Justin Bolinger University of Maine - Main Follow this and additional works at: https://digitalcommons.library.umaine.edu/honors Part of the Comparative and Evolutionary Physiology Commons, Genomics Commons, and the Molecular Genetics Commons Recommended Citation Bolinger, Justin, "A Comparative Genomics Approach to Using High-Throughput Gene Expression Data to Study Limb Regeneration in Ambystoma Mexicanum and Danio Rerio: Developing a More Completely Annotated Database" (2013). Honors College. 116. https://digitalcommons.library.umaine.edu/honors/116 This Honors Thesis is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Honors College by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. A COMPARATIVE GENOMICS APPROACH TO USING HIGH-THROUGHPUT GENE EXPRESSION DATA TO STUDY LIMB REGENERATION IN AMBYSTOMA MEXICANUM AND DANIO RERIO: DEVELOPING A MORE COMPLETELY ANNOTATED DATABASE by Justin Bolinger A Thesis Submitted in Partial Fulfillment of the Requirements for a Degree with Honors (Chemical Engineering) The Honors College University of Maine at Orono May 2013 Advisory Committee: Keith Hutchison, Department of Biochemistry and Molecular Biology, Advisor Benjamin King, Staff Scientist, Mount Desert Island Biological Laboratory, co-Advisor John Hwalek, Department of Chemical Engineering François Amar, Department of Chemistry Kevin Roberge, Department of Physics and Astronomy ABSTRACT Axolotl (Ambystoma mexicanum) and the zebrafish (Danio rerio) represent organisms extensively studied because of their remarkable capability of fully regenerating completely functional tissues after a traumatic event takes place. -

Genome Wide Methylome Alterations in Lung Cancer
RESEARCH ARTICLE Genome Wide Methylome Alterations in Lung Cancer Nandita Mullapudi1☯, Bin Ye2☯, Masako Suzuki3, Melissa Fazzari3, Weiguo Han1, Miao K. Shi1, Gaby Marquardt1, Juan Lin4, Tao Wang5, Steven Keller6, Changcheng Zhu7, Joseph D. Locker7¤, Simon D. Spivack1,3,5* 1 Department of Medicine/Pulmonary, Albert Einstein College of Medicine, Bronx, New York, United States of America, 2 Department of Bioinformatics, Albert Einstein College of Medicine, Bronx, New York, United States of America, 3 Department of Genetics, Albert Einstein College of Medicine, Bronx, New York, United States of America, 4 Department of Epidemiology & Population Health, Division of Biostatistics, Albert Einstein College of Medicine, Bronx, New York, United States of America, 5 Department of Epidemiology & Population Health, Albert Einstein College of Medicine, Bronx, New York, United States of America, 6 Department of Cardiovascular &Thoracic Surgery, Montefiore Medical Center, Bronx, New York, United States of America, a11111 7 Department of Pathology, Montefiore Medical Center, Bronx, New York, United States of America ☯ These authors contributed equally to this work. ¤ Current address: Department of Pathology, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, United States of America * [email protected] OPEN ACCESS Abstract Citation: Mullapudi N, Ye B, Suzuki M, Fazzari M, Han W, Shi MK, et al. (2015) Genome Wide Aberrant cytosine 5-methylation underlies many deregulated elements of cancer. Among Methylome Alterations in -

Consistent Biomarkers and Related Pathogenesis Underlying Asthma Revealed by Systems Biology Approach
International Journal of Molecular Sciences Article Consistent Biomarkers and Related Pathogenesis Underlying Asthma Revealed by Systems Biology Approach 1, 1, 1 1 2 3 Xiner Nie y, Jinyi Wei y, Youjin Hao , Jingxin Tao , Yinghong Li , Mingwei Liu , Boying Xu 1 and Bo Li 1,* 1 College of Life Sciences, Chongqing Normal University, Chongqing 401331, China 2 School of Biological Information, Chongqing University of Posts and Telecommunications, Chongqing 400065, China 3 College of Laboratory Medicine, Chongqing Medical University, Chongqing 400046, China * Correspondence: [email protected] These authors contributed equally to this work. y Received: 21 July 2019; Accepted: 17 August 2019; Published: 19 August 2019 Abstract: Asthma is a common chronic airway disease worldwide. Due to its clinical and genetic heterogeneity, the cellular and molecular processes in asthma are highly complex and relatively unknown. To discover novel biomarkers and the molecular mechanisms underlying asthma, several studies have been conducted by focusing on gene expression patterns in epithelium through microarray analysis. However, few robust specific biomarkers were identified and some inconsistent results were observed. Therefore, it is imperative to conduct a robust analysis to solve these problems. Herein, an integrated gene expression analysis of ten independent, publicly available microarray data of bronchial epithelial cells from 348 asthmatic patients and 208 healthy controls was performed. As a result, 78 up- and 75 down-regulated genes were identified