Saurashtra University Re – Accredited Grade ‘B’ by NAAC (CGPA 2.93)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Narrating North Gujarat: a Study of Amrut Patel's
NARRATING NORTH GUJARAT: A STUDY OF AMRUT PATEL’S CONTRIBUTION TO FOLK LITERATURE A MINOR RESEARCH PROJECT :: SUBMITTED TO :: UNIVERSITY GRANTS COMMISSION :: SUBMITTED BY :: DR.RAJESHKUMAR A. PATEL ASSOCIATE PROFESSOR SMT.R.R.H.PATEL MAHILA ARTS COLLEGE, VIJAPUR DIST.MEHSANA (GUJARAT) 2015 Preface Literature reflects human emotions, thoughts and expressions. It’s a record of activities and abstract ideas of human beings. The oral tradition of literature is the aspect of literature passing ideas and feelings mouth to mouth. I’ve enjoyed going through the precious and rare pieces of folk literature collected and edited by Amrut Patel. I congratulate and salute Amrut Patel for rendering valuable service to this untouchable, vanishing field of civilization. His efforts to preserve the vanishing forms of oral tradition stand as milestone for future generation and students of folk literature. I am indebted to UGC for sanctioning the project. The principal of my college, Dr.Sureshbhai Patel and collegues have inspired me morally and intellectually. I thank them. I feel gratitude to Nanabhai Nadoda for uploding my ideas and making my work easy. Shaileshbhai Paramar, the librarian has extended his time and help, I thank him. Shri Vishnubhai M.Patel, Shri R.R.Ravat, Shri.D.N.Patel, Shri S.M.Patel, Shri R.J.Brahmbhatt, Shri J.J.Rathod., Shri D.S.Kharadi, B.L.Bhangi and Maheshbhai Limbachiya have suppoted me morally. I thank them all. DR.Rajeshkumar A.Patel CONTENTS 1. Introduction: 1.1 North Gujarat 1.2 Life and Works of Dr.Amrut Patel 1.3 Folk Literature-An Overview 2. -

Tamarind 1990 - 2004
Tamarind 1990 - 2004 Author A. K. A. Dandjouma, C. Tchiegang, C. Kapseu and R. Ndjouenkeu Title Ricinodendron heudelotii (Bail.) Pierre ex Pax seeds treatments influence on the q Year 2004 Source title Rivista Italiana delle Sostanze Grasse Reference 81(5): 299-303 Abstract The effects of heating Ricinodendron heudelotii seeds on the quality of the oil extracted was studied. The seeds were preheated by dry and wet methods at three temperatures (50, 70 and 90 degrees C) for 10, 20, 30 and 60 minutes. The oil was extracted using the Soxhlet method with hexane. The results showed a significant change in oil acid value when heated at 90 degrees C for 60 minutes, with values of 2.76+or-0.18 for the dry method and 2.90+or-0.14 for the wet method. Heating at the same conditions yielded peroxide values of 10.70+or-0.03 for the dry method and 11.95+or-0.08 for the wet method. Author A. L. Khandare, U. Kumar P, R. G. Shanker, K. Venkaiah and N. Lakshmaiah Title Additional beneficial effect of tamarind ingestion over defluoridated water supply Year 2004 Source title Nutrition Reference 20(5): 433-436 Abstract Objective: We evaluated the effect of tamarind (Tamarindus indicus) on ingestion and whether it provides additional beneficial effects on mobilization of fluoride from the bone after children are provided defluoridated water. Methods: A randomized, diet control study was conducted in 30 subjects from a fluoride endemic area after significantly decreasing urinary fluoride excretion by supplying defluoridated water for 2 wk. -

Utilization of Lignocellulosic Waste from Bidi Industry for Removal of Dye from Aqueous Solution
Int. J. Environ. Res., 2(4): 385-390, Autumn 2008 ISSN: 1735-6865 Utilization of Lignocellulosic Waste from Bidi Industry for Removal of Dye from Aqueous Solution Nagda, G. K.* and Ghole, V. S. Department of Environmental Sciences, University of Pune, Pune, India Received 26 Oct 2007; Revised 15 March 2008; Accepted 15 April 2008 ABSTRACT: A new, local agro-industrial waste was valorized by chemical treatment and tested for its ability to remove cationic dye from aqueous solution. Tendu (Diospyros melanoxylon) leaves refuse, a solid waste from bidi industry which caused disposal problem, was studied as a biosorbent. Raw tendu waste (TLR), along with sulfuric acid carbonized tendu waste (TLR-CM) and tendu waste treated with dilute sulfuric acid (TLR-2N) were utilized as sorbent for uptake of crystal violet from aqueous solutions. Adsorption studies were carried out at various dye concentrations and contact times. It followed the pseudo-second-order kinetics and followed the Langmuir adsorption isotherm. Interestingly, milder acid treatment of the tendu waste enhanced biosorption, whereas drastic acid carbonization of tendu waste resulted in reduced adsorption of dye. The maximum adsorption capacities for crystal violet for TLR-2N, TLR and TLR-CM are 67.57, 42.92 and 22.47 mg/g respectively. Commercial activated carbon had maximum adsorption capacity for crystal violet of 151.52 mg/g. Thus a renewable solid waste with mild acid treatment can offer a cost effective alternative to activated carbon. Key words: Biosorption, Diospyros melanoxylon, Solid tendu waste, Dye, Crystal violet INTRODUCTION Colored wastewater is a consequence of synthetic textile dyes because of the chemical industrial processes both in the dye manufacturing stability of these pollutants (Anjaneyulu et al., industries and in the dye-consuming industries. -

Wild Life Sanctuaries in INDIA
A M K RESOURCE WORLD GENERAL KNOWLEDGE www.amkresourceinfo.com Wild Life Sanctuaries in INDIA Wildlife Sanctuaries in India are 441 in number. They are a home to hundreds and thousands of various flora and fauna. A wide variety of species thrive in such Wildlife Sanctuaries. With the ever growing cement – jungle, it is of utmost importance to protect and conserve wildlife and give them their own, natural space to survive Wildlife Sanctuaries are established by IUCN category II protected areas. A wildlife sanctuary is a place of refuge where abused, injured, endangered animals live in peace and dignity. Senchal Game Sanctuary. Established in 1915 is the oldest of such sanctuaries in India. Chal Batohi, in Gujarat is the largest Wildlife Sanctuary in India. The conservative measures taken by the Indian Government for the conservation of Tigers was awarded by a 30% rise in the number of tigers in 2015. According to the Red Data Book of International Union for Conservation of Nature (IUCN), there are 47 critically endangered species in India. DO YOU KNOW? Wildlife sanctuaries in India are established by IUCN category II protected areas. India has 537 wildlife sanctuaries referred to as wildlife sanctuaries category IV protected areas. Among these, the 50 tiger reserves are governed by Project Tiger, and are of special significance in the conservation of the tiger. Some wildlife sanctuaries in India are specifically named bird sanctuary, e.g., Keoladeo National Park before attaining National Park status. Many of them being referred as as a particular animal such as Jawai leopard sanctuary in Rajasthan. -

Notes and Comments on the Distribution of Two Endemic Lygosoma Skinks (Squamata: Scincidae: Lygosominae) from India
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 December 2014 | 6(14): 6726–6732 Note The family Scincidae is the Notes and comments on the distribution largest group among lizards, of two endemic Lygosoma skinks comprising more than 1558 species (Squamata: Scincidae: Lygosominae) ISSN 0974-7907 (Online) (Uetz & Hosek 2014). Of the from India ISSN 0974-7893 (Print) seven subfamilies recognized, the subfamily Lygosominae contains Raju Vyas OPEN ACCESS over 52 species in five genera (Uetz & Hosek 2014). The genus 505, Krishnadeep Tower, Mission Road, Fatehgunj, Vadodara, Gujarat Lygosoma Hardwicke & Gray, 1827 has a long and 390002, India [email protected] complicated nomenclatural history (see Geissler et al. 2011). In India, the genus Lygosoma is represented by nine species, of which five are endemic (Datta-Roy et al. 2014), including Günther’s Supple Skink Lygosoma City, Vadodara District and after examination both the guentheri (Peters, 1879) and the Lined Supple Skink skinks were released in the nearby riverine habitat of Lygosoma lineata (Gray, 1839). These are less studied, Vishwamitri River within the limits of the city area. terrestrial, insectivorous and diurnal supple-skinks Lygosoma guentheri: On 12 December 2013, a large (Molur & Walker 1998). Both these species are found adult specimen of Lygosoma (Image 1) was captured by in peninsular India and are classified ‘Least Concern’ a local rescue group from a garden in Vadodara City, species by the IUCN Red List of Threatened Species Gujarat. The specimen was identified as L. guentheri (Srinivasulu & Srinivasulu 2013a, b). with the help of the literature (Boulenger 1890; Smith Reserved forest and degraded areas of the northern 1935). -

Patterns of Discovery of Birds in Kerala Breeding of Black-Winged
Vol.14 (1-3) Jan-Dec. 2016 newsletter of malabar natural history society Akkulam Lake: Changes in the birdlife Breeding of in two decades Black-winged Patterns of Stilt Discovery of at Munderi Birds in Kerala Kadavu European Bee-eater Odonates from Thrissur of Kadavoor village District, Kerala Common Pochard Fulvous Whistling Duck A new duck species - An addition to the in Kerala Bird list of - Kerala for subscription scan this qr code Contents Vol.14 (1-3)Jan-Dec. 2016 Executive Committee Patterns of Discovery of Birds in Kerala ................................................... 6 President Mr. Sathyan Meppayur From the Field .......................................................................................................... 13 Secretary Akkulam Lake: Changes in the birdlife in two decades ..................... 14 Dr. Muhamed Jafer Palot A Checklist of Odonates of Kadavoor village, Vice President Mr. S. Arjun Ernakulam district, Kerala................................................................................ 21 Jt. Secretary Breeding of Black-winged Stilt At Munderi Kadavu, Mr. K.G. Bimalnath Kattampally Wetlands, Kannur ...................................................................... 23 Treasurer Common Pochard/ Aythya ferina Dr. Muhamed Rafeek A.P. M. A new duck species in Kerala .......................................................................... 25 Members Eurasian Coot / Fulica atra Dr.T.N. Vijayakumar affected by progressive greying ..................................................................... 27 -
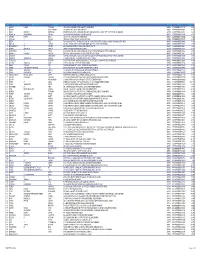
Section 124- Unpaid and Unclaimed Dividend
Sr No First Name Middle Name Last Name Address Pincode Folio Amount 1 ASHOK KUMAR GOLCHHA 305 ASHOKA CHAMBERS ADARSHNAGAR HYDERABAD 500063 0000000000B9A0011390 36.00 2 ADAMALI ABDULLABHOY 20, SUKEAS LANE, 3RD FLOOR, KOLKATA 700001 0000000000B9A0050954 150.00 3 AMAR MANOHAR MOTIWALA DR MOTIWALA'S CLINIC, SUNDARAM BUILDING VIKRAM SARABHAI MARG, OPP POLYTECHNIC AHMEDABAD 380015 0000000000B9A0102113 12.00 4 AMRATLAL BHAGWANDAS GANDHI 14 GULABPARK NEAR BASANT CINEMA CHEMBUR 400074 0000000000B9A0102806 30.00 5 ARVIND KUMAR DESAI H NO 2-1-563/2 NALLAKUNTA HYDERABAD 500044 0000000000B9A0106500 30.00 6 BIBISHAB S PATHAN 1005 DENA TOWER OPP ADUJAN PATIYA SURAT 395009 0000000000B9B0007570 144.00 7 BEENA DAVE 703 KRISHNA APT NEXT TO POISAR DEPOT OPP OUR LADY REMEDY SCHOOL S V ROAD, KANDIVILI (W) MUMBAI 400067 0000000000B9B0009430 30.00 8 BABULAL S LADHANI 9 ABDUL REHMAN STREET 3RD FLOOR ROOM NO 62 YUSUF BUILDING MUMBAI 400003 0000000000B9B0100587 30.00 9 BHAGWANDAS Z BAPHNA MAIN ROAD DAHANU DIST THANA W RLY MAHARASHTRA 401601 0000000000B9B0102431 48.00 10 BHARAT MOHANLAL VADALIA MAHADEVIA ROAD MANAVADAR GUJARAT 362630 0000000000B9B0103101 60.00 11 BHARATBHAI R PATEL 45 KRISHNA PARK SOC JASODA NAGAR RD NR GAUR NO KUVO PO GIDC VATVA AHMEDABAD 382445 0000000000B9B0103233 48.00 12 BHARATI PRAKASH HINDUJA 505 A NEEL KANTH 98 MARINE DRIVE P O BOX NO 2397 MUMBAI 400002 0000000000B9B0103411 60.00 13 BHASKAR SUBRAMANY FLAT NO 7 3RD FLOOR 41 SEA LAND CO OP HSG SOCIETY OPP HOTEL PRESIDENT CUFFE PARADE MUMBAI 400005 0000000000B9B0103985 96.00 14 BHASKER CHAMPAKLAL -

Review Profile of Mahal Village an Eco-Tourism Planning Proposal
International Journal of Management, Technology And Engineering ISSN NO : 2249-7455 Review Profile of Mahal Village an Eco-tourism Planning Proposal 1 2 Zinal Nirishbhai Patel , Sejal S. Bhagat 1Post Graduate Student, Town and Country Planning, Sarvajanik College of Engineering and Technology (Surat, Gujarat, India) 2 Assistant Professor, Faculty of Civil Engineering, Sarvajanik College of Engineering andTechnology (Surat, Gujarat, India) ABSTRACT As tourism is one of the fastest growing industries today, thus within the tourism industry events are getting more and more important Events can offer various economic and social benefits for destinations, and therefore destination managers can and should employ events effectively in a tourism role. Even around hugely popular tourist places, there lie a number of attractive, but less known places, when a tourist from another country visits a highly popular tourists spot in India, his/her sightseeing is limited to a maximum of two days. Eco Tourism in India, In short, ecotourism can be categorized as a tourism programmer that is - "Nature based, ecologically sustainable, where education and interpretation is a major constituent and where local people are benefited." All this together can be called eco-tourism. Mahal village has a scope of recorded, normal and social possibilities. The advertising procedures arranged with the intend to make and build up Mahal tourism and at what stage is the picture of Mahal is the topic of this paper. The aim of the this paper is To analyze Tourism Potential of Dang District and to Frame Circuit based planning proposal of Mahal as an Eco-tourism the objectives are To study existing Tourism scenario of the area & identify potential and issues of Mahal spot for Eco-tourism purpose. -

Ncris2019-Abstract.Pdf
Message from Y S Rajan (Padma Shri Awardee) RK University, Rajkot has taken up a very vital topic of relevance to Indian economy and society as a whole, in choosing to hold a national conference on the subject of Scientific Research and Commercialization. The benefits derived from such an orientation, are not just limited to academic institutions alone. If one studies the history of scientific, engineering, medical, industrial and even some military applications of developed countries, their academic institutions have played great innovative roles. In India somehow, the dominant approach has been to place scientific research as an anti-thesis to commercial applications. One can see that the number of patents held by Nobel laureates are on rise world- wide. Also, a number of excellent scientific works has been done by those who work on commercially oriented projects. A wide range of speakers in this conference would show the way for our students who can make a difference for themselves and India with a positive attitude to research and commercialization. India will benefit in the process making it a powerful lead country in the world. I commend the organizers. -Y. S. Rajan Message I am extremely pleased to learn that School of Science, RK University is organizing a two days National Conference on Recent Innovations in Science under the banner NCRIS – 2019 on 18th and 19th January 2019. Gujarat Council on Science and Technology (GUJCOST) Govt. of Gujarat and CSIR Govt. of India has sponsored it. Moreover I am very much delighted on the initiative taken by School of Science to host innovations in science for students to translate into inventions of India like recent path breaking invention of Hydroelectric Cell which is accepted globally. -

Saurashtra University Rajkot Answered Questions Get the Latest Answers on Cutoff, Courses, Placements, Admission, Fees, Ranking & Eligibility
Saurashtra University Rajkot An exclusive Guide by Saurashtra University Rajkot Answered questions Get the latest answers on cutoff, courses, placements, admission, fees, ranking & eligibility. All answers have been submitted by students, alumni & experts. I want to admission & dublicate results of BsC home science 1 Answer . 15 Views PRADEEP KUMAR a year ago Scholar-Level 18 Sr. Section Eng r(Desig n) Please write about the course name in which you wants to take the a dmission for further details. For duplicate result you have to approa ch to your College/ University. Further there are various links are avail able on University website (https://www.saurashtrauniversity.edu/) fr om where you can download the desired exam result. I am an BBA sem 4 student of saurashtra university. I want to take transf er to Gujarat University f or SEM 5 af ter completing Sem 4.Possible? 1 Answer . 1 Follower . 29 Views HEENA AGRAWAL a year ago Scholar-Level 16 H try to g ive best solution.. Hi, Disclaimer: This PDF is auto-generated based on the information available on Shiksha as on 24-Sep-2021. It is sometimes possible to move straight from one university to ano ther, usually in the first few weeks of your first year, or between year s if the courses are similar enough. If you've decided this is the right thing for you, these are the steps you need to take (although things might work differently depending on your university.) Contact the university you want to move to. You need to find out wh ether they will accept you onto the course. -

Groundwater Brochure the Dang District Gujarat
For Official Use Technical Report Series GROUNDWATER BROCHURE THE DANG DISTRICT GUJARAT Compiled by B.K.Gupta Scientist – C Government of India Ministry of Water Resources Central Ground Water Board West Central Region Ahmedabad March, 2014 THE DANG DISTRICT AT A GLANCE Sl.No. Items Statistics 1 GENERAL INFORMATION i) Geographical area as per state territory/as per village papers 1764 (Sq. Km) ii) Administrative Divisions (As on 3/2010) : Number of Talukas/ One / Number of villages/ No of villages having drinking water facility as on 1.04.2009 311/311 iii) Populations (As on 2011 census) 227000 Population density 126/sq.km iv) Average Annual Rainfall (mm) (1951to 1980) 1928, normal annual rain fall (mm), 2011 1635 2 GEOMORPHOLOGY Major Physiographic Units: Deccan Trap country, terraced topography with flat topped conical hills, Small plateau and steep sided narrow valleys. ( RL between 105 to 1317 m above sea level) Major Drainages: Purna, Ambika, Khapri , Gira and Ghogha 3 MAJOR SOIL TYPES: Lateritic soils, deep black clayey and loamy soils and red sandy soils. 4 NUMBERS OF GROUND WATER MONITORING WELLS CGWB (As on 31-03-2012) No of Dug Wells 25 No of Piezometers 2 5 PREDOMINANT GEOLOGICAL FORMATIONS: Deccan trap basalt with dykes. 6 HYDROGEOLOGY Major Water Bearing Formations: Deccan trap basalt with dikes and alluvium. Pre- monsoon depth to water level ( May 2012) 2.89m (Jakhana) to 12.38 m ( Mheskatri) Post- monsoon depth to water level (November 2012) 0.50m (Jakhana/ChinchPada/Ghubita) to 9.55m (Mhesktri) The seasonal ( Pre-Post -

Eurylaimides Species Tree
Eurylaimides ⋆Velvet Asity, Philepitta castanea Schlegel’s Asity, Philepitta schlegeli ⋆ Philepittidae Common Sunbird-Asity, Neodrepanis coruscans Yellow-bellied Sunbird-Asity, Neodrepanis hypoxantha ⋆Grauer’s Broadbill, Pseudocalyptomena graueri ⋆Long-tailed Broadbill, Psarisomus dalhousiae ⋆ Eurylaimidae Dusky Broadbill, Corydon sumatranus Visayan Broadbill, Sarcophanops samarensis ⋆Wattled Broadbill, Sarcophanops steerii ⋆Silver-breasted Broadbill, Serilophus lunatus ⋆Black-and-red Broadbill, Cymbirhynchus macrorhynchos ⋆Banded Broadbill, Eurylaimus javanicus Black-and-yellow Broadbill, Eurylaimus ochromalus Gray-headed Broadbill, Smithornis sharpei Rufous-sided Broadbill, Smithornis rufolateralis Smithornithidae ⋆African Broadbill, Smithornis capensis Hose’s Broadbill, Calyptomena hosii ⋆Green Broadbill, Calyptomena viridis Calyptomenidae Whitehead’s Broadbill, Calyptomena whiteheadi ⋆Sapayoa, Sapayoa aenigma:0.1 Sapayoidae Blue-banded Pitta, Erythropitta arquata Garnet Pitta, Erythropitta granatina Graceful Pitta, Erythropitta venusta Black-crowned Pitta, Erythropitta ussheri Erythropitta Whiskered Pitta, Erythropitta kochi Philippine Pitta, Erythropitta erythrogaster Sula Pitta, Erythropitta dohertyi Sulawesi Pitta, Erythropitta celebensis Sangihe Pitta, Erythropitta caeruleitorques Siao Pitta, Erythropitta palliceps South Moluccan Pitta, Erythropitta rubrinucha North Moluccan Pitta, Erythropitta rufiventris Louisiade Pitta, Erythropitta meeki ⋆Papuan Pitta, Erythropitta macklotii Bismarck Pitta, Erythropitta novaehibernicae Pittidae