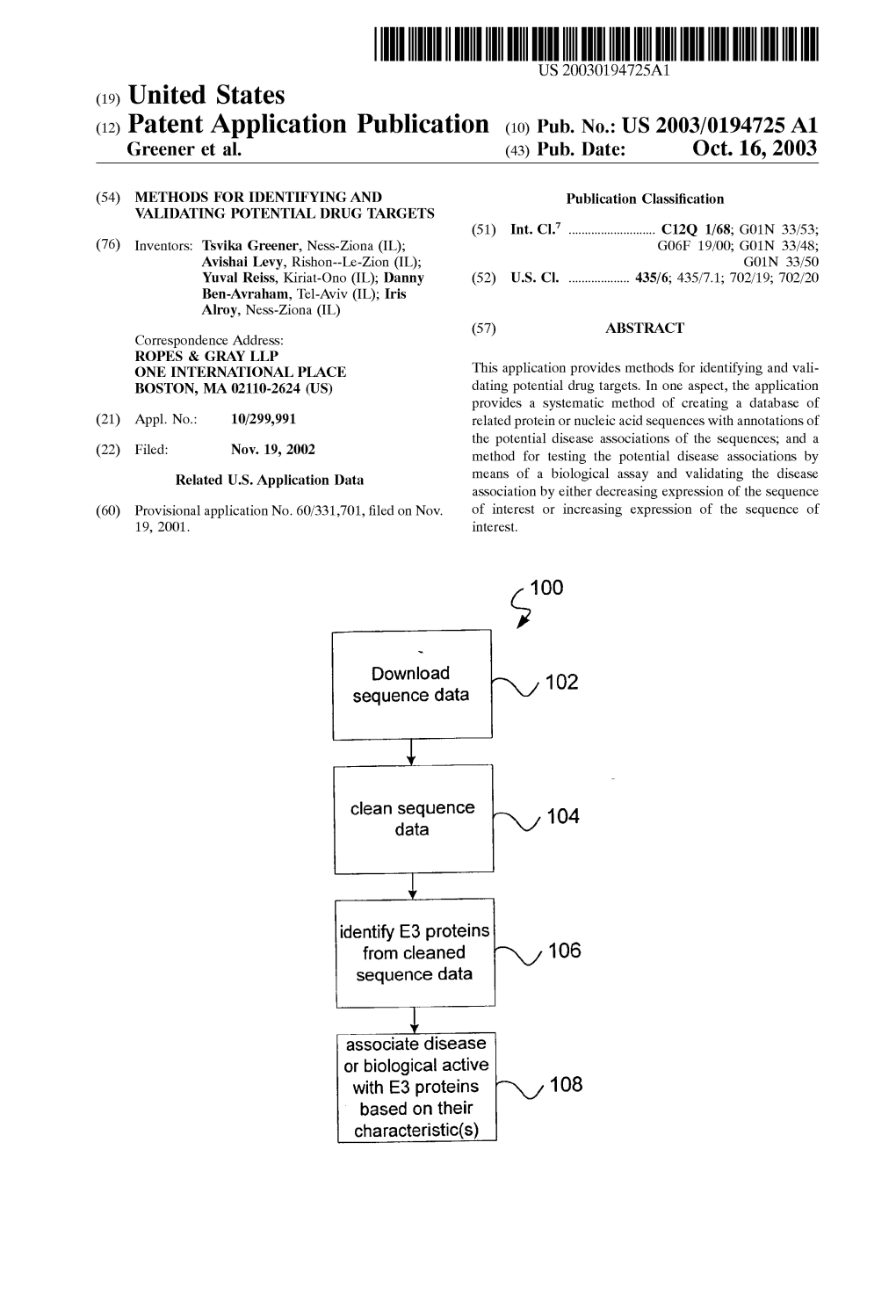
(12) Patent Application Publication (10) Pub. No.: US 2003/0194725A1 Greener Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Title of the Article
Mechanism-Anchored Profiling Derived from Epigenetic Networks Predicts Outcome in Acute Lymphoblastic Leukemia Xinan Yang, PhD1, Yong Huang, MD1, James L Chen, MD1, Jianming Xie, MSc2, Xiao Sun, PhD2, Yves A Lussier, MD1,3,4§ 1Center for Biomedical Informatics and Section of Genetic Medicine, Department of Medicine, The University of Chicago, Chicago, IL 60637 USA 2State Key Laboratory of Bioelectronics, Southeast University, 210096 Nanjing, P.R.China 3The University of Chicago Cancer Research Center, and The Ludwig Center for Metastasis Research, The University of Chicago, Chicago, IL 60637 USA 4The Institute for Genomics and Systems Biology, and the Computational Institute, The University of Chicago, Chicago, IL 60637 USA §Corresponding author Email addresses: XY: [email protected] YH: [email protected] JC: [email protected] JX: [email protected] XS: [email protected] YL: [email protected] - 1 - Abstract Background Current outcome predictors based on “molecular profiling” rely on gene lists selected without consideration for their molecular mechanisms. This study was designed to demonstrate that we could learn about genes related to a specific mechanism and further use this knowledge to predict outcome in patients – a paradigm shift towards accurate “mechanism-anchored profiling”. We propose a novel algorithm, PGnet, which predicts a tripartite mechanism-anchored network associated to epigenetic regulation consisting of phenotypes, genes and mechanisms. Genes termed as GEMs in this network meet all of the following criteria: (i) they are co-expressed with genes known to be involved in the biological mechanism of interest, (ii) they are also differentially expressed between distinct phenotypes relevant to the study, and (iii) as a biomodule, genes correlate with both the mechanism and the phenotype. -

Regulation and Evolutionary Origins of Repulsive
REGULATION AND EVOLUTIONARY ORIGINS OF REPULSIVE GUIDANCE MOLECULE C / HEMOJUVELIN EXPRESSION: A MUSCLE-ENRICHED GENE INVOLVED IN IRON METABOLISM by Christopher John Severyn B.A. (University of California, Berkeley) 2002 A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in BIOCHEMISTRY AND MOLECULAR BIOLOGY Presented to the Department of Biochemistry & Molecular Biology OREGON HEALTH & SCIENCE UNIVERSITY School of Medicine July 2010 Department of Biochemistry & Molecular Biology, School of Medicine OREGON HEALTH & SCIENCE UNIVERSITY _________________________________________________ CERTIFICATE OF APPROVAL _________________________________________________ This is to certify that the Ph.D. dissertation of Christopher J. Severyn has been approved by the following: __________________________ Peter Rotwein, M.D., dissertation advisor Professor of Biochemistry and Department Chair __________________________ Maureen Hoatlin, Ph.D., committee chair Associate Professor of Biochemistry __________________________ Matt Thayer, Ph.D., committee member Professor of Biochemistry __________________________ Ujwal Shinde, Ph.D., committee member Professor of Biochemistry __________________________ Jim Lundblad, M.D., Ph.D., committee member Associate Professor of Medicine and Chief of Endocrinology TABLE OF CONTENTS Page List of Tables iii List of Figures iv List of Abbreviations vii Acknowledgements xiv Publications arising from this Dissertation xvi Abstract xvii Key words xix Chapter 1: Introduction 1. 1 General Overview 2 1. 2 Expression and Regulation of RGMc 3 1. 3 Gene Regulation: Transcription from an evolutionary perspective 4 1. 4 Gene Regulation: Translation 6 1. 5 Iron Metabolism in Eukaryotes 6 1. 6 Dissertation Overview 7 Chapter 2: Repulsive Guidance Molecule Family 2. 1 Summary 15 2. 2 Introduction 16 2. 3 RGMa 16 2. 4 RGMb 21 2. -

Cross-Modulatory Actions of Cell Cycle Machinery on Estrogen Receptor-Α Level and Transcriptional Activity in Breast Cancer Cells
CROSS-MODULATORY ACTIONS OF CELL CYCLE MACHINERY ON ESTROGEN RECEPTOR-α LEVEL AND TRANSCRIPTIONAL ACTIVITY IN BREAST CANCER CELLS BY SHWETA BHATT DISSERTATION Submitted In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Biochemistry in the Graduate College of the University of Illinois at Urbana-Champaign, 2010 Urbana, Illinois Doctoral Committee: Professor Benita S. Katzenellenbogen (Chair) Professor David J. Shapiro Professor Milan K. Bagchi Professor Paul J. Hergenrother THESIS ABSTRACT Breast cancer is one of the most highly diagnosed cancers in women and the second largest cause of death of women in United States. The anti-estrogen tamoxifen which blocks gene expression through estradiol bound ERα, and hence the growth stimulatory effects of estradiol, has been widely used for decades for treating patients with ERα positive or hormone dependent breast cancer. Despite its obvious benefits, in as high as 40% of the patients receiving tamoxifen therapy there is an eventual relapse of the disease largely due to acquired resistance to the drug, underlying mechanism for which is rather poorly understood. Elucidating the molecular basis underlying “acquired tamoxifen resistance” and agonistic effects of tamoxifen on cellular growth was the primary focus of my doctoral research. We addressed this by two approaches, one being studying the molecular mechanism for the regulation of cellular levels of ERα so as to prevent its loss in ERα positive or restore its levels in ERα negative breast cancers and second to investigate the role of tamoxifen in modulating the expression of ERα target genes independent of estradiol as a function of its stimulatory or estrogenic effects on breast cancer cell growth. -

ARTICLE Complex Inheritance Pattern Resembling Autosomal Recessive Inheritance Involving a Microdeletion in Thrombocytopenia– Absent Radius Syndrome
ARTICLE Complex Inheritance Pattern Resembling Autosomal Recessive Inheritance Involving a Microdeletion in Thrombocytopenia– Absent Radius Syndrome Eva Klopocki,* Harald Schulze,* Gabriele Strauß, Claus-Eric Ott, Judith Hall, Fabienne Trotier, Silke Fleischhauer, Lynn Greenhalgh, Ruth A. Newbury-Ecob, Luitgard M. Neumann, Rolf Habenicht, Rainer Ko¨nig, Eva Seemanova, Andre´ Megarbane, Hans-Hilger Ropers, Reinhard Ullmann, Denise Horn, and Stefan Mundlos Thrombocytopenia–absent radius (TAR) syndrome is characterized by hypomegakaryocytic thrombocytopenia and bi- lateral radial aplasia in the presence of both thumbs. Other frequent associations are congenital heart disease and a high incidence of cow’s milk intolerance. Evidence for autosomal recessive inheritance comes from families with several affected individuals born to unaffected parents, but several other observations argue for a more complex pattern of inheritance. In this study, we describe a common interstitial microdeletion of 200 kb on chromosome 1q21.1 in all 30 investigated patients with TAR syndrome, detected by microarray-based comparative genomic hybridization. Analysis of the parents revealed that this deletion occurred de novo in 25% of affected individuals. Intriguingly, inheritance of the deletion along the maternal line as well as the paternal line was observed. The absence of this deletion in a cohort of control individuals argues for a specific role played by the microdeletion in the pathogenesis of TAR syndrome. We hypothesize that TAR syndrome is associated with a deletion on chromosome 1q21.1 but that the phenotype develops only in the presence of an additional as-yet-unknown modifier (mTAR). Thrombocytopenia–absent radius (TAR) syndrome (MIM Cow’s milk allergy or intolerance appears to be relatively 274000) is a clinically well-characterized malformation common among patients with TAR syndrome and may syndrome. -

Open Data for Differential Network Analysis in Glioma
International Journal of Molecular Sciences Article Open Data for Differential Network Analysis in Glioma , Claire Jean-Quartier * y , Fleur Jeanquartier y and Andreas Holzinger Holzinger Group HCI-KDD, Institute for Medical Informatics, Statistics and Documentation, Medical University Graz, Auenbruggerplatz 2/V, 8036 Graz, Austria; [email protected] (F.J.); [email protected] (A.H.) * Correspondence: [email protected] These authors contributed equally to this work. y Received: 27 October 2019; Accepted: 3 January 2020; Published: 15 January 2020 Abstract: The complexity of cancer diseases demands bioinformatic techniques and translational research based on big data and personalized medicine. Open data enables researchers to accelerate cancer studies, save resources and foster collaboration. Several tools and programming approaches are available for analyzing data, including annotation, clustering, comparison and extrapolation, merging, enrichment, functional association and statistics. We exploit openly available data via cancer gene expression analysis, we apply refinement as well as enrichment analysis via gene ontology and conclude with graph-based visualization of involved protein interaction networks as a basis for signaling. The different databases allowed for the construction of huge networks or specified ones consisting of high-confidence interactions only. Several genes associated to glioma were isolated via a network analysis from top hub nodes as well as from an outlier analysis. The latter approach highlights a mitogen-activated protein kinase next to a member of histondeacetylases and a protein phosphatase as genes uncommonly associated with glioma. Cluster analysis from top hub nodes lists several identified glioma-associated gene products to function within protein complexes, including epidermal growth factors as well as cell cycle proteins or RAS proto-oncogenes. -

SZDB: a Database for Schizophrenia Genetic Research
Schizophrenia Bulletin vol. 43 no. 2 pp. 459–471, 2017 doi:10.1093/schbul/sbw102 Advance Access publication July 22, 2016 SZDB: A Database for Schizophrenia Genetic Research Yong Wu1,2, Yong-Gang Yao1–4, and Xiong-Jian Luo*,1,2,4 1Key Laboratory of Animal Models and Human Disease Mechanisms of the Chinese Academy of Sciences and Yunnan Province, Kunming Institute of Zoology, Kunming, China; 2Kunming College of Life Science, University of Chinese Academy of Sciences, Kunming, China; 3CAS Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Shanghai, China 4YGY and XJL are co-corresponding authors who jointly directed this work. *To whom correspondence should be addressed; Kunming Institute of Zoology, Chinese Academy of Sciences, Kunming, Yunnan 650223, China; tel: +86-871-68125413, fax: +86-871-68125413, e-mail: [email protected] Schizophrenia (SZ) is a debilitating brain disorder with a Introduction complex genetic architecture. Genetic studies, especially Schizophrenia (SZ) is a severe mental disorder charac- recent genome-wide association studies (GWAS), have terized by abnormal perceptions, incoherent or illogi- identified multiple variants (loci) conferring risk to SZ. cal thoughts, and disorganized speech and behavior. It However, how to efficiently extract meaningful biological affects approximately 0.5%–1% of the world populations1 information from bulk genetic findings of SZ remains a and is one of the leading causes of disability worldwide.2–4 major challenge. There is a pressing -

BAC Array CGH in Patients with Velocardiofacial Syndrome-Like
BMC Medical Genetics BioMed Central Research article Open Access BAC array CGH in patients with Velocardiofacial syndrome-like features reveals genomic aberrations on chromosome region 1q21.1 Anna Brunet1,2, Lluís Armengol1, Damià Heine3, Jordi Rosell3, Manel García- Aragonés1, Elisabeth Gabau2, Xavier Estivill*1,4 and Miriam Guitart*2 Address: 1Genes and Disease Program, CIBER en Epidemiología y Salud Pública (CIBERESP), Center for Genomic Regulation (CRG), Barcelona, Catalonia, Spain, 2Genetic laboratory UDIAT-Centre Diagnòstic, Fundació Parc Taulí - Institut Universitari UAB, Corporació Sanitària Parc Taulí, Sabadell, Catalonia, Spain, 3Hospital Universitari Son Dureta, Mallorca, Spain and 4Pompeu Fabra University (UPF), Barcelona, Catalonia, Spain Email: Anna Brunet - [email protected]; Lluís Armengol - [email protected]; Damià Heine - [email protected]; Jordi Rosell - [email protected]; Manel García-Aragonés - [email protected]; Elisabeth Gabau - [email protected]; Xavier Estivill* - [email protected]; Miriam Guitart* - [email protected] * Corresponding authors Published: 23 December 2009 Received: 14 January 2009 Accepted: 23 December 2009 BMC Medical Genetics 2009, 10:144 doi:10.1186/1471-2350-10-144 This article is available from: http://www.biomedcentral.com/1471-2350/10/144 © 2009 Brunet et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: Microdeletion of the chromosome 22q11.2 region is the most common genetic aberration among patients with velocardiofacial syndrome (VCFS) but a subset of subjects do not show alterations of this chromosome region. -

Supplementary Materials and Tables a and B
SUPPLEMENTARY MATERIAL 1 Table A. Main characteristics of the subset of 23 AML patients studied by high-density arrays (subset A) WBC BM blasts MYST3- MLL Age/Gender WHO / FAB subtype Karyotype FLT3-ITD NPM status (x109/L) (%) CREBBP status 1 51 / F M4 NA 21 78 + - G A 2 28 / M M4 t(8;16)(p11;p13) 8 92 + - G G 3 53 / F M4 t(8;16)(p11;p13) 27 96 + NA G NA 4 24 / M PML-RARα / M3 t(15;17) 5 90 - - G G 5 52 / M PML-RARα / M3 t(15;17) 1.5 75 - - G G 6 31 / F PML-RARα / M3 t(15;17) 3.2 89 - - G G 7 23 / M RUNX1-RUNX1T1 / M2 t(8;21) 38 34 - + ND G 8 52 / M RUNX1-RUNX1T1 / M2 t(8;21) 8 68 - - ND G 9 40 / M RUNX1-RUNX1T1 / M2 t(8;21) 5.1 54 - - ND G 10 63 / M CBFβ-MYH11 / M4 inv(16) 297 80 - - ND G 11 63 / M CBFβ-MYH11 / M4 inv(16) 7 74 - - ND G 12 59 / M CBFβ-MYH11 / M0 t(16;16) 108 94 - - ND G 13 41 / F MLLT3-MLL / M5 t(9;11) 51 90 - + G R 14 38 / F M5 46, XX 36 79 - + G G 15 76 / M M4 46 XY, der(10) 21 90 - - G NA 16 59 / M M4 NA 29 59 - - M G 17 26 / M M5 46, XY 295 92 - + G G 18 62 / F M5 NA 67 88 - + M A 19 47 / F M5 del(11q23) 17 78 - + M G 20 50 / F M5 46, XX 61 59 - + M G 21 28 / F M5 46, XX 132 90 - + G G 22 30 / F AML-MD / M5 46, XX 6 79 - + M G 23 64 / M AML-MD / M1 46, XY 17 83 - + M G WBC: white blood cell. -

Developpement D'outils Bioinformatiques Et Statistiques Pour L'analyse Du Transcriptome Hepatique Humain Par Puces A
Developpement d’outils bioinformatiques et statistiques pour l’analyse du transcriptome hepatique humain par puces a ADN : application a la reponse inflammatoire aigue systemique. Gregory Lefebvre To cite this version: Gregory Lefebvre. Developpement d’outils bioinformatiques et statistiques pour l’analyse du transcrip- tome hepatique humain par puces a ADN : application a la reponse inflammatoire aigue systemique.. Sciences du Vivant [q-bio]. Université de Rouen, 2006. Français. tel-00182773 HAL Id: tel-00182773 https://tel.archives-ouvertes.fr/tel-00182773 Submitted on 28 Oct 2007 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. No d’ordre: 00000 THESE` pr´esent´ee devant l’Universit´ede Rouen pour obtenir le grade de : Docteur de l’Universite´ de Rouen Mention Biologie moleculaire´ par Gr´egory LEFEBVRE Equipe´ d’accueil : INSERM - Unit´e519 Ecole´ Doctorale : Chimie-Biologie de Haute-Normandie Titre de la th`ese : D´eveloppement d’outils bioinformatiques et statistiques pour l’analyse du transcriptome h´epatique humain par puces `aADN : application `ala r´eponse inflammatoire aigu¨esyst´emique. soutenue le 17 novembre 2006 devant la commission d’examen compos´ee de : M. le Pr Fran¸cois Tron Pr´esident M. -

1 Novel Expression Signatures Identified by Transcriptional Analysis
ARD Online First, published on October 7, 2009 as 10.1136/ard.2009.108043 Ann Rheum Dis: first published as 10.1136/ard.2009.108043 on 7 October 2009. Downloaded from Novel expression signatures identified by transcriptional analysis of separated leukocyte subsets in SLE and vasculitis 1Paul A Lyons, 1Eoin F McKinney, 1Tim F Rayner, 1Alexander Hatton, 1Hayley B Woffendin, 1Maria Koukoulaki, 2Thomas C Freeman, 1David RW Jayne, 1Afzal N Chaudhry, and 1Kenneth GC Smith. 1Cambridge Institute for Medical Research and Department of Medicine, Addenbrooke’s Hospital, Hills Road, Cambridge, CB2 0XY, UK 2Roslin Institute, University of Edinburgh, Roslin, Midlothian, EH25 9PS, UK Correspondence should be addressed to Dr Paul Lyons or Prof Kenneth Smith, Department of Medicine, Cambridge Institute for Medical Research, Addenbrooke’s Hospital, Hills Road, Cambridge, CB2 0XY, UK. Telephone: +44 1223 762642, Fax: +44 1223 762640, E-mail: [email protected] or [email protected] Key words: Gene expression, autoimmune disease, SLE, vasculitis Word count: 2,906 The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Annals of the Rheumatic Diseases and any other BMJPGL products to exploit all subsidiary rights, as set out in their licence (http://ard.bmj.com/ifora/licence.pdf). http://ard.bmj.com/ on September 29, 2021 by guest. Protected copyright. 1 Copyright Article author (or their employer) 2009. -

Comparative Analysis of the Ubiquitin-Proteasome System in Homo Sapiens and Saccharomyces Cerevisiae
Comparative Analysis of the Ubiquitin-proteasome system in Homo sapiens and Saccharomyces cerevisiae Inaugural-Dissertation zur Erlangung des Doktorgrades der Mathematisch-Naturwissenschaftlichen Fakultät der Universität zu Köln vorgelegt von Hartmut Scheel aus Rheinbach Köln, 2005 Berichterstatter: Prof. Dr. R. Jürgen Dohmen Prof. Dr. Thomas Langer Dr. Kay Hofmann Tag der mündlichen Prüfung: 18.07.2005 Zusammenfassung I Zusammenfassung Das Ubiquitin-Proteasom System (UPS) stellt den wichtigsten Abbauweg für intrazelluläre Proteine in eukaryotischen Zellen dar. Das abzubauende Protein wird zunächst über eine Enzym-Kaskade mit einer kovalent gebundenen Ubiquitinkette markiert. Anschließend wird das konjugierte Substrat vom Proteasom erkannt und proteolytisch gespalten. Ubiquitin besitzt eine Reihe von Homologen, die ebenfalls posttranslational an Proteine gekoppelt werden können, wie z.B. SUMO und NEDD8. Die hierbei verwendeten Aktivierungs- und Konjugations-Kaskaden sind vollständig analog zu der des Ubiquitin- Systems. Es ist charakteristisch für das UPS, daß sich die Vielzahl der daran beteiligten Proteine aus nur wenigen Proteinfamilien rekrutiert, die durch gemeinsame, funktionale Homologiedomänen gekennzeichnet sind. Einige dieser funktionalen Domänen sind auch in den Modifikations-Systemen der Ubiquitin-Homologen zu finden, jedoch verfügen diese Systeme zusätzlich über spezifische Domänentypen. Homologiedomänen lassen sich als mathematische Modelle in Form von Domänen- deskriptoren (Profile) beschreiben. Diese Deskriptoren können wiederum dazu verwendet werden, mit Hilfe geeigneter Verfahren eine gegebene Proteinsequenz auf das Vorliegen von entsprechenden Homologiedomänen zu untersuchen. Da die im UPS involvierten Homologie- domänen fast ausschließlich auf dieses System und seine Analoga beschränkt sind, können domänen-spezifische Profile zur Katalogisierung der UPS-relevanten Proteine einer Spezies verwendet werden. Auf dieser Basis können dann die entsprechenden UPS-Repertoires verschiedener Spezies miteinander verglichen werden. -

ARTICLE Complex Inheritance Pattern Resembling Autosomal Recessive Inheritance Involving a Microdeletion in Thrombocytopenia– Absent Radius Syndrome
ARTICLE Complex Inheritance Pattern Resembling Autosomal Recessive Inheritance Involving a Microdeletion in Thrombocytopenia– Absent Radius Syndrome Eva Klopocki,* Harald Schulze,* Gabriele Strauß, Claus-Eric Ott, Judith Hall, Fabienne Trotier, Silke Fleischhauer, Lynn Greenhalgh, Ruth A. Newbury-Ecob, Luitgard M. Neumann, Rolf Habenicht, Rainer Ko¨nig, Eva Seemanova, Andre´ Megarbane, Hans-Hilger Ropers, Reinhard Ullmann, Denise Horn, and Stefan Mundlos Thrombocytopenia–absent radius (TAR) syndrome is characterized by hypomegakaryocytic thrombocytopenia and bi- lateral radial aplasia in the presence of both thumbs. Other frequent associations are congenital heart disease and a high incidence of cow’s milk intolerance. Evidence for autosomal recessive inheritance comes from families with several affected individuals born to unaffected parents, but several other observations argue for a more complex pattern of inheritance. In this study, we describe a common interstitial microdeletion of 200 kb on chromosome 1q21.1 in all 30 investigated patients with TAR syndrome, detected by microarray-based comparative genomic hybridization. Analysis of the parents revealed that this deletion occurred de novo in 25% of affected individuals. Intriguingly, inheritance of the deletion along the maternal line as well as the paternal line was observed. The absence of this deletion in a cohort of control individuals argues for a specific role played by the microdeletion in the pathogenesis of TAR syndrome. We hypothesize that TAR syndrome is associated with a deletion on chromosome 1q21.1 but that the phenotype develops only in the presence of an additional as-yet-unknown modifier (mTAR). Thrombocytopenia–absent radius (TAR) syndrome (MIM common among patients with TAR syndrome and may 274000) is a clinically well-characterized malformation provoke eosinophilia.2 Patients with TAR syndrome typi- syndrome.