Medical Physics. Ivan Tanev Ivanov. Thracian University. 2016
CHAPTER 5. HIGH FREQUENCY ELECTROMAGNETIC FIELD
5.1. Spectrum of electromagnetic radiation. Generation and biological effects of electromagnetic radiation
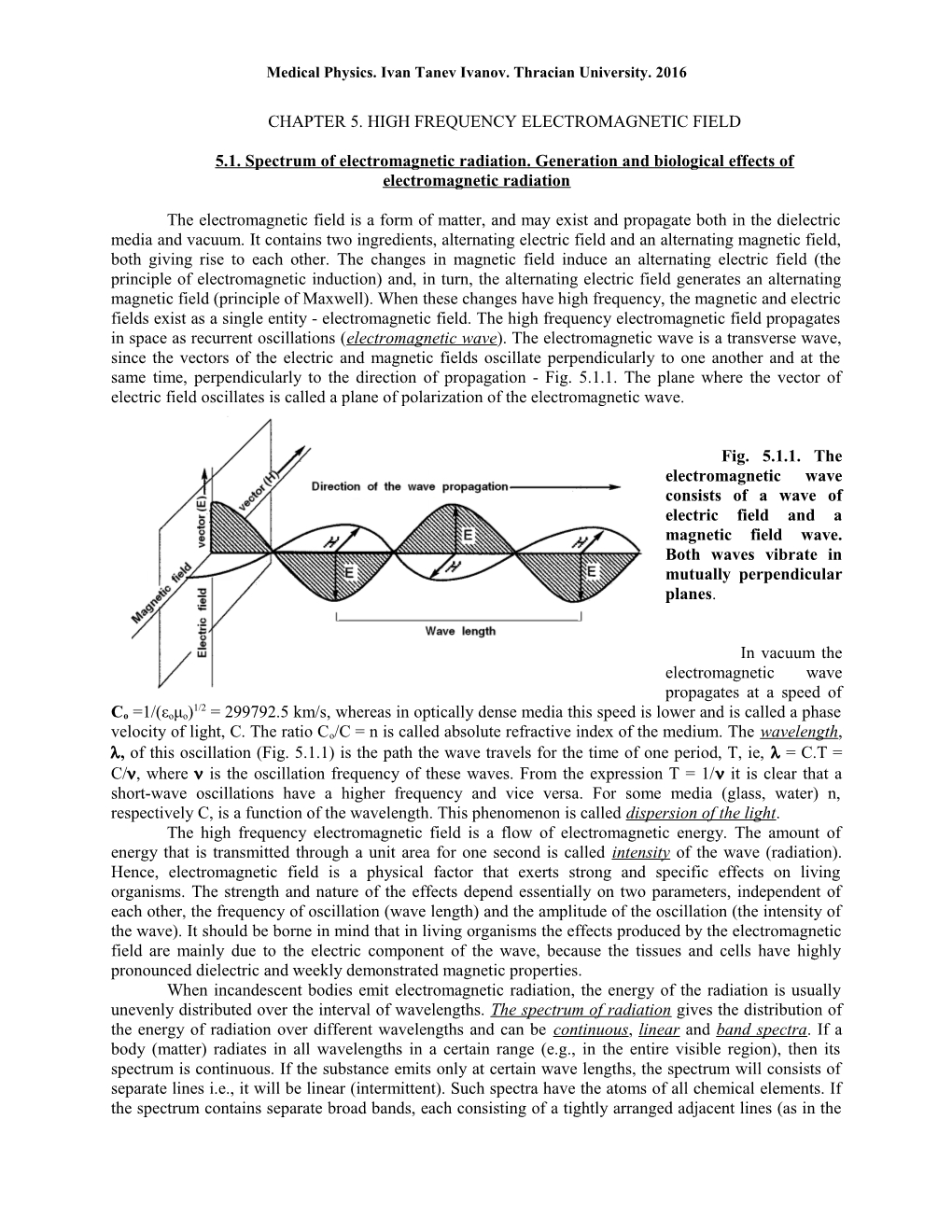
The electromagnetic field is a form of matter, and may exist and propagate both in the dielectric media and vacuum. It contains two ingredients, alternating electric field and an alternating magnetic field, both giving rise to each other. The changes in magnetic field induce an alternating electric field (the principle of electromagnetic induction) and, in turn, the alternating electric field generates an alternating magnetic field (principle of Maxwell). When these changes have high frequency, the magnetic and electric fields exist as a single entity - electromagnetic field. The high frequency electromagnetic field propagates in space as recurrent oscillations (electromagnetic wave). The electromagnetic wave is a transverse wave, since the vectors of the electric and magnetic fields oscillate perpendicularly to one another and at the same time, perpendicularly to the direction of propagation - Fig. 5.1.1. The plane where the vector of electric field oscillates is called a plane of polarization of the electromagnetic wave.
Fig. 5.1.1. The electromagnetic wave consists of a wave of electric field and a magnetic field wave. Both waves vibrate in mutually perpendicular planes.
In vacuum the electromagnetic wave propagates at a speed of 1/2 Со =1/(εoμo) = 299792.5 km/s, whereas in optically dense media this speed is lower and is called a phase velocity of light, C. The ratio Co/C = n is called absolute refractive index of the medium. The wavelength, , of this oscillation (Fig. 5.1.1) is the path the wave travels for the time of one period, T, ie, = C.T = C/, where is the oscillation frequency of these waves. From the expression T = 1/ it is clear that a short-wave oscillations have a higher frequency and vice versa. For some media (glass, water) n, respectively C, is a function of the wavelength. This phenomenon is called dispersion of the light. The high frequency electromagnetic field is a flow of electromagnetic energy. The amount of energy that is transmitted through a unit area for one second is called intensity of the wave (radiation). Hence, electromagnetic field is a physical factor that exerts strong and specific effects on living organisms. The strength and nature of the effects depend essentially on two parameters, independent of each other, the frequency of oscillation (wave length) and the amplitude of the oscillation (the intensity of the wave). It should be borne in mind that in living organisms the effects produced by the electromagnetic field are mainly due to the electric component of the wave, because the tissues and cells have highly pronounced dielectric and weekly demonstrated magnetic properties. When incandescent bodies emit electromagnetic radiation, the energy of the radiation is usually unevenly distributed over the interval of wavelengths. The spectrum of radiation gives the distribution of the energy of radiation over different wavelengths and can be continuous, linear and band spectra. If a body (matter) radiates in all wavelengths in a certain range (e.g., in the entire visible region), then its spectrum is continuous. If the substance emits only at certain wave lengths, the spectrum will consists of separate lines i.e., it will be linear (intermittent). Such spectra have the atoms of all chemical elements. If the spectrum contains separate broad bands, each consisting of a tightly arranged adjacent lines (as in the Medical Physics. Ivan Tanev Ivanov. Thracian University. 2016 continuous spectrum), it is a band spectra. Such spectra have substances made of molecules. If the molecules are small the bands are narrow and with sharp borders. Substances made up of large molecules have spectrum containing large bands with fuzzy edges. Depending on the wavelength, , the spectrum of electromagnetic waves is divided into different ranges - radio waves, infrared rays, visible light, ultraviolet waves, X-rays and gamma-rays (Fig. 5.1.2). Of all the electromagnetic waves only those with between 380 and 760 nm are visible to the human eye and are known as visible light. In this narrow frequency area, however, we differentiate 6 basic color areas and a large number of intermediate color hues. All other electromagnetic radiations are invisible to the eye and can be detected only by means of appropriate technical means (detectors). The presented figure indicates that such different by their properties rays as radio, infra-red (IR) light, visible light, ultraviolet (UV) rays, X-rays and -rays have the same nature - these are oscillations (waves) of the electromagnetic field. In general, the ability of electromagnetic radiation to penetrate the tissues of human body strongly diminishes with decreasing the wavelength. An important exception to this rule are the X-rays and gamma rays which penetrate much deeper than UV- rays and the rays of visible light. The radio waves from the meter, decimeter and centimeter range are used in radar, radio and television transmitters and mobile phones. The dielectric media and human tissues slightly absorb these types of electromagnetic waves. Such waves are emitted by all conductive media including metal conductors when alternating electric current flows through them. They are usually generated in oscillating circuits composed of capacitor and inductor (coil). Radio waves with very high intensity produce heat in human tissues. At low intensity, this radiation increases the temperature of tissues with no more than 0.1°C, nevertheless, it induce various effects, referred to as non-thermal effects. They include; increase in the permeability of plasma membranes, inhibition of the activity of enzymes and protein channels, influence on immunocompetent cells and on reproduction of bacteria, influence on the flow of Ca2+ ions in the brain tissue coupled to concequent change in the rhythm of brain potentials and functions of the cerebral cortex, effects on DNA. Very often these effects are frequency dependent. In human they cause fatigue, insomnia, forgetfulness and weakened memory. When the amplitude of these waves is less than a certain threshold the above effects can not occur.
Fig. 5.1.2. Scale (specter) of electromagnetic waves.
Mobile phones emit electromagnetic waves with frequencies from 450 to 1900 MHz and can be a potential risk to large groups of people. This risk is based on several mechanisms for non-thermal effects of electromagnetic radiation. One such mechanism is due to the resonance absorption of electromagnetic radiation by biological macromolecules and biomembranes which have natural frequencies of vibration in this range. For example, in biomembranes polar heads of phospholipids perform rotary motions with a frequency of about 109 Hz, the characteristic frequencies of the bound water are in the range 108-109 Hz, and these of the free water are of the order of 1010 Hz. The area of 1010 - 1011 Hz contains the characteristic - + oscillations of some functional groups like COO , NH3 , playing an important role in the functioning of protein molecules. These are frequencies in the microwave range of electromagnetic radiation and therefore biomacromolecules and biomembranes can resonancely absorb the energy of this radiation. Medical Physics. Ivan Tanev Ivanov. Thracian University. 2016
Microwave radiation represents millimeter radio waves and has a large capability to penetrate into the tissues of the human body (10 - 20 cm). It is used in the treatment of deeply localized inflammatory and other disorders to heat the internal tissues with high water content. The strongest heating is induced in tissues containing a lot of water. Water strongly absorbs microwave radiation due to the high value of its dielectric permittivity. Infrared rays are emitted from the hot bodies and, therefore, they are also referred to as heat radiation. The higher the body temperature, the greater is the intesity of heat radiation, and the rays have a shorter wavelength. The infrared rays very efficiently heat the bodies which absorb them. Their penetration in human tissues, however, is small (2-3 cm). Together with their thermal radiation, highly heated bodies emit also visible and even ultraviolet light. In addition, visible and ultraviolet lights are emitted through luminescence whereas some kind of energy, other than heat, excites atoms and molecules of the body. The exited atoms and molecules release their energy by light emittion. Visible and UV radiation are used in medicine for phototherapy and photodynamic therapy. Their penetration in the tissues and skin, however, is very small, only about 1 mm. In photochemistry, visible and ultraviolet light is used to create a photographic image, to initiate fusion reactions and polymerization. Reontgen, or X-rays, are generated by slowing down fast-moving charged particles. In medicine they are produced in the X-ray tubes and in linear accelerators of electrons. Unlike the long-wave UV radiation and visible light, X-rays have abnormal, extremely high ability to penetrate into bodies and in human tissues. This penetration capacity is used to obtain images of the internal parts of the bodies, internal organs of the human body in medicine; internal defects of various products in defectoscopy; at the border check of passengers' luggage.
Fig. 5.1.3. Frequency profile (spectrum) of the absorption of electromagnetic radiation by Earth's atmosphere.
Gamma rays are produced only during the radioactive decay of unstable nuclei of certain isotopes. These rays as well as short-wave X-rays have a highly damaging effect on the structure of the bodies as they ionize and excite atoms and molecules and produce free radicals. In chemotherapy these effects are used to kill the cancerous tissues. In industry these rays are applied in the non-destructive gamma ray defectoscopy. Shortwave radiation (gamma rays, X-rays and ultraviolet rays) falling on a certain type of substances (luminophors) elicits luminescence, that is emission of visible light. Directing these types of radiation to a screen covered with a luminophor induces luminescence allowing them to become visible to human eye. Medical Physics. Ivan Tanev Ivanov. Thracian University. 2016
The sun is a powerful source of electromagnetic radiation, which delivers an energy flux with density of about 1100 W/m2 to the Earth's surface perpendicular to the beam of propagation. It emits all types of electromagnetic radiation, listed above, although mostly intense is its radiation in the yellow- green interval of the visible range, where the sensitivity of human eye is greatest. However, the Sun radiation is largely absorbed by the Earth atmosphere, depending on the wavelength (Fig. 5.1.3). The gamma-rays, X-rays and hard UV rays, which are harmful to the life on Earth, are absorbed by the ozone layer of the Earth's atmosphere. The Earth's atmosphere also absorbs most of the heat radiation allowing a temperature, acceptable for the living organisms on the Earth's surface. Only a part of the thermal radiation, radio waves, the rays of the visible range and soft UV rays can penetrate to the Earth's surface. Electromagnetic rays having a single frequency are referred to as monochromatic (of the same color). If two monochromatic waves have the same phase angles, they are called coherent waves. This means that these waves change consistently over time. Coherent waves demonstrate the phenomenon called interference (superposition). When two coherent waves fall and overlay on a small surface, their electric field vectors add or subtract to each other, depending on their phase angle difference. Thereat, the amplitude of the resultant electric field is increased in some areas of the surface and decreased or eliminated in the adjacent areas. This interference explains the fact that electromagnetic waves (e.g. visible light), coming out of a small hole, spread further as a straight line beam. The propagation in the form of beem is typical of the short-wave radiation - infrared, visible and ultraviolet, all designated as optical radiation. Yet another phenomenon, diffraction, is typical for the electromagnetic waves. When such a wave encounters a barrier with dimensions, L, much larger than its wavelength (L ), the wave is reflected and does not penetrate behind the barrier. When the size of the barrier is comparable to , (i.e., L ), the wave circumvents the barrier; this is called diffraction of the wave. Interferention and diffraction are inherent of each wave process. The fact that electromagnetic radiations, including light, also demonstrate such phenomena proves that light and other electromagnetic radiations are waves. However, electromagnetic radiations, especially shortwave ones, elicit effects that are characteristic for the flow of particles. Such effect, for example, is the photoelectric effect – ejection of valence electrons when the atoms are irradiated with visible light. Based on experimental data, quantum mechanics formulates that each electromagnetic wave can be regarded as a stream of particles (corpuscles, microvolumes with the size of ), in which the energy of the electric field is concentrated. Any such corpuscle, called photon of electromagnetic radiation or electromagnetic quantum, has the energy E = h., according to the equation of the German physicist Max Planck. Here h is Planck's constant, h = 6.626196 x10-34 J s. The monochromatic electromagnetic field carries an energy flow, which is equal to the number of photons crossing a surface of 1 m2, multiplied by the mean energy of the individual photons. The strongest corpuscular character is exhibited by the high frequencies radiations - ultraviolet, X-ray and, in particular, the gamma-rays. Their photons have enough energy to break chemical bonds in molecules, to produce photoelectric effect, to excite and ionize the atoms and molecules. Hence, these types of radiation are called ionizing radiations. The main factor determining the type of the effects elicited by the radiation is the energy of individual photons, i.e., the frequency of the electromagnetic wave. In turn the number of absorbed photons, i.e., the intensity of the wave determines the amplitude of the arised effect. Radiation whose photons have not enough energy (visible light, infrared light, microwaves and radiowaves) are referred to as non-ionizing. Whether or not the electromagnetic radiation will be regarded as waves or a stream of particles depends on what object they interact with. If the dimensions of the object are much larger than , the electromagnetic radiation behaves like waves. If the dimensions of the objects are close to or smaller than , the radiations behave like a stream of particles. Interestingly, the corpuscular streams of elementary particles - electrons, protons, and many others, exhibit similar dual character. Based on experimental facts the quantum mechanics formulates that each elementary particle exhibits properties of a wave with = h/ (mv) (wave of Louis de Broglie). Here, m is the mass of the particle, and v is its speed. This conception is Medical Physics. Ivan Tanev Ivanov. Thracian University. 2016 used in the electron microscope and in the newer proton microscope. In these instruments a flow of electrons and protons is used to obtain magnified image of micro bioobjects with space resolution as small as the of the Louis de Broglie’wave of these particles.
5.2. Infrared and ultraviolet light. Sources and detectors of infrared and ultraviolet light. Effects of infrared and ultraviolet light on hu man s . Medical applications of infrared and ultraviolet light s
Light (optical radiation) is absorbed strongly by the tissues, hence, it exerts many biological effects. During its absorption by the atoms and molecules, the light behaves as a flow of particles (photons), each having an energy E = h = hc/. Absorption of a photon by an atom or molecule is a resonance process, i.e., a given type of atoms or molecules can only absorb photons having a particular frequency (energy). Upon its absorption the photon disappears and its energy is absorbed entirely by the atom or molecule. On the other hand, the atom (molecule) increases its energy and shifts into a new, short- lived "excited" state. Depending on the amount of energy absorbed, this new state is expressed by the change of the mechanical motions (vibration, rotation) of the molecule or by the change in the state of its valence electron. This can lead to a variety of biological effects, whose type depends on the frequency (energy) of the absorbed photons. The strength of the effect depends on the number of atoms or molecules affected, i.e., the number of absorbed photons (the intensity of light). In general, photons of infrared light have low frequency and low energy. The absorption of such photons changes only the mechanical energy of the molecules, i.e., the energy of the oscillation and rotation of molecules, but not the state of valence electrons. As a consequence, these photons can not cause photochemical reactions. The energy of the absorbed infrared light is converted into thermal energy (heat) of atoms and molecules. Thus, the infrared rays can only heat the bodies which absorb them. The range of infrared (IR) radiation is arbitrarily subdivided into near ( between 0.67 - 2.5 m), medium ( between 2.5-50 m) and distant ( > 50 m) ranges. The heat radiation of hot bodies is composed mainly of IR-light and partly of visible light. Depending on the temperature of the body, the maximum of radiation is in the near or in the middle region of infrared rays. For example, the incandescent lamps with heated filament (T = 2500°C) emit in the near IR region and partially in the visible region. Kanthal heating-resistors, heated by an electric current to about 500 - 600°C emit mostly in the middle IR region. Other sources of infrared rays used in medicine are halogen and mercury lamps with high pressure and gas laser s whose active medium is a gas (CO2, argon, etc.). The IR radiation is strongly absorbed by water and penetrates a few centimeters only into the human tissues with high water content. IR rays exercise only thermal effect on tissues. They heat up the surface layer of human body, triggering the thermoregulatory system, hence, the blood circulation is accelerated, transport and metabolic processes are enhanced. These outcoms explain the healing and tempering effect of the sauna in which a person is placed in a hot (150°C) and humid atmosphere for a short time (10 min). This causes an increase in the internal body temperature to 38-39°C, then the man rapidly immerses in cold water. IR radiation emitted by warm, large surfaces, mounted in the living rooms, determines the thermal comfort of inhabitants in them. Thermography is completely harmless, non-invasive method for imaging, which measures the temperature distribution over the surface of human body, particularly of superficial blood vessels. For this purpose a television camera is used that captures infrared rays of superfacial tissues and creates an image whose colors correspond to the temperature of the outside tissues. The wave length, , of visible and particularly ultraviolet (UV) lightis is smaller and therefore the frequency and energy of the photons is significantly greater. The absorbance of such photons changes the electronic state of atoms and molecules whereat the valence electrons are displaced away from the nucleus. Such an excited molecule is in a chemically-activated state, hence, it is highly reactive. As a Medical Physics. Ivan Tanev Ivanov. Thracian University. 2016 consequence, the exited molecules may give rise to a large number of photochemical reactions - photosynthesis, photodecomposition (generation of free radicals, ions), photooxidation, photohydration, photoisomerization. Intense visible light is used for the treatment of jaundice in infants, whereat the toxic pigment bilirubin accumulates in their blood during the first days after birth. The pigment strongly absorbs light with between 420 to 480 nm resulting in its degradation and detoxification. In this type of therapy (phototherapy), the sick newborn is placed in a bath of bright light for 12-24 hours. The UV light has shorter wave length, respectively it contains photons with even higher energy. These photons are absorbed in the upper layer of the skin, where they cause a variety of photochemical reactions, culminating in a biological response. The UV photons cause harmful effects which can be classified as acute and chronic. The acute effects are caused by the exposure to long wave UV light and are both short-lived and reversible. These effects include mainly sunburn (or erythema) and tanning (or pigment darkening). The chronic effects of UV exposure can be much more serious, even life threatening, and include premature aging of the skin, suppression of the immune system, damage to the eyes, and skin cancer. Depending on the wave length (the energy of its photons) the UV-radiation is subdivided into three zones; UV-A, UV-B and UV-C zones: 1) UV-A zone (anti-rachitis zone) occupies the interval 380-315 nm. Their photons have moderately high energy (soft UV-light) and when they fall onto the skin, they cause the synthesis of anti- rachitis hormone (vitamin D), sex hormones, melanin and others. The vitamin D deficiency is the main cause for rickets as it is required for proper calcium absorption from guts. Sunlight, especially ultraviolet light, helps human skin cells convert vitamin D from an inactive to active state. 2) UV-B zone (erythematous zone) occupies the range 315-280 nm. Their photons have higher energy and create free radicals in tissues that oxidize lipids of cell membranes, thus causing burn of the skin (erythema), premature aging (loss in elasticity) and suppression of the immune system. Particularly sensitive is the eye cornea as more than 99% of UV radiation is absorbed by the front of the eyes, causing inflammation and cataracts. In smaller doses, the UV-B light exhibits a therapeutic tempering effect due mainly to the synthesis of melanin (tanning of skin). In turn, the melanin has an antioxidative effect destroying free radicals. Melanin has a protective effect because it absorbs a part of the UV rays, especially those having a shorter wavelength. 3) UV-C or bactericidal area) occupies the interval 280-200 nm. These photons have high energy (hard UV radiation) and can denature proteins and nucleic acids. UV radiation with specific wavelength (approximately 260 nm) is selectively absorbed by the nucleobases of the nucleic acids and raises the valence electrons to higher orbits. Cytosine and thymine are the most sensitive nucleobases to this effect of UV rays. Under the influence of UV light, two adjacent thymine bases may be crosslinked into a dimer. This produces cytotoxic, bactericidal and mutagenic effects. Such UV rays are used for sterilization of air in larger rooms as well as of tools and utensils. In human they cause mutations and carcinogenesis. UV light is used to detect the presence of fluorescent material deposited on the skin at certain skin diseases, for example in fungal skin diseases. In the so called photodynamic therapy of skin tumors the UV light is used in combination with appropreate photosensitising agents. Similarly, in the treatment of psoriasis 8-methoxypsoralen is the photosensitising agent whereat the entire surface of the skin is repeatedly irradiated with UV light. Sun is a natural source of IR and UV rays. About 50% of its radiation represents infrared rays and 10% is UV-light. Earth's atmosphere has an ozone layer, located about 30-50 km above the earth surface.
This layer contains ozone, О3, which is produced through the decay of oxygen molecules under the action . . . of UV-rays according to the reactions: 1) О2 → О + О and 2) О + О2 → О3. Ozone absorbs the short wave UV rays coming from the Sun thereby preventing photochemical damage to living organisms on Earth. During the industrial activities, however, hydrogen chlorides are emitted, which destroy ozone molecules in the ozone layer. Medical Physics. Ivan Tanev Ivanov. Thracian University. 2016
In medicine mercury lamps are used as artificial source of UV rays. They consist of a glass tube made up of quartz glass (the ordinary glass absorbs UV rays!). The tube is filled with a gas and contains several mercury drops on its botom. Electric current is passed through the gas (gas discharge) which generates heat evaporating the mercury drops. The electric current compels the gas ions to move with high speeds. The gas ions collide with the mercury atoms and exite them causing their luminescence. The spectrum of mercury lamp contains a large number of lines in the UV region and in the visible region. The energy of light can be measured by the induced blackening of photographic plates. More frequently, it is messured using various types of detectors : 1) Heat detectors. When the detector absorbs light, for example IR light, its temperature increases. This leads to a change in the electrical resistance of the detector (in bolometers) or to a generation of electric voltage (in thermocouples). These variables are measured and calibrated in the units of light energy flow. 2) Photoelectric detectors. The absorption of a photon by some substances causes the release of valence electrons (photoelectric effect). The ejected electrons reduce the electrical resistance (in photocells and photoresistors) or generate an electric voltage (in photoelements) all proportional to the luminous flux. The most sensitive detector of this type is the photoelectron multiplier tube (FEM), which can register individual photons. These detectors comprise a photocathode, a large number of intermediate electrodes (dinods) and a final anode (Fig. 5.2.1). An accelerating electric field is imposed in the space between the cathode and anode. Based on the mechanism of external photoeffect the incident photon knocks out an electron from the photocathode. The emited electron is accelerated by the field and knocks out several additional electrons from the first dinod. These electrons in turn knock out more additional electrons from the second dinod and so on. Thus, a huge avalanche forms while all electrons reach the anode.
Fig. 5.2.1. Scheme of a photoelectron multiplier tube (left) and of electron-optical converter (right).
3) The photoelectric effect is used in the so called electron-optical converters (EOC) - Fig.5.2.1, which uses the infrared light emitted by hot bodies for their observation (thermal imaging). Infrared rays are focused and form an image on the photocathode. Each photon knocks out photoelectrons from the photocathode. The emited photoelectrons are accelerated by the electric field of the accelerating electrode and finally focused on the luminescent screen. This causes luminescence of the screen (cathodoluminiscence), forming a magnified image of the observed object. This converter is used in X-ray apparatus (fluorographs) as image magnifier, in binoculars for night vision, in the apparatus for thermography imaging.