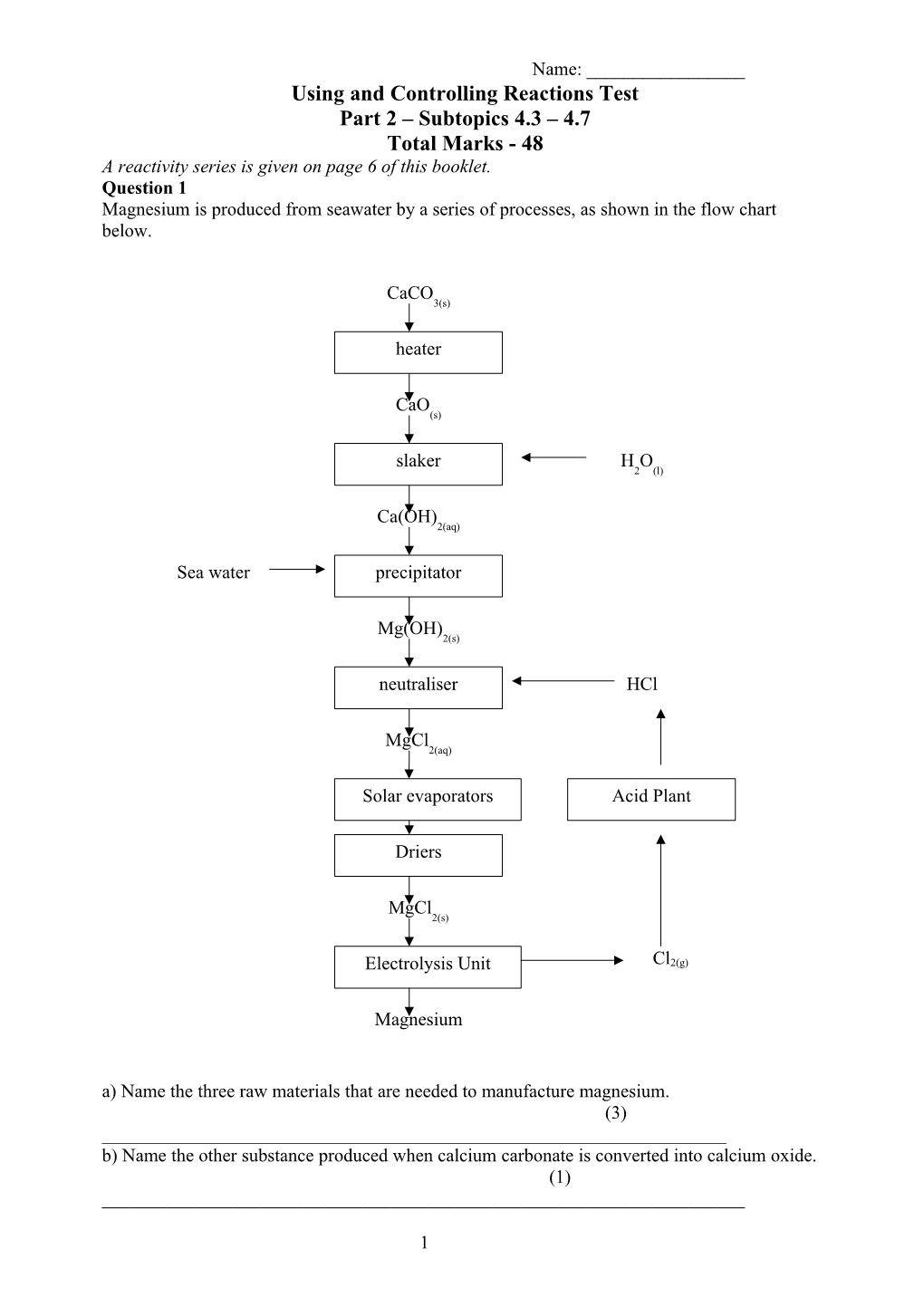
Name: ______Using and Controlling Reactions Test Part 2 – Subtopics 4.3 – 4.7 Total Marks - 48 A reactivity series is given on page 6 of this booklet. Question 1 Magnesium is produced from seawater by a series of processes, as shown in the flow chart below.
CaCO 3(s)
heater
CaO (s)
slaker H O 2 (l)
Ca(OH) 2(aq)
Sea water precipitator
Mg(OH) 2(s)
neutraliser HCl
MgCl 2(aq)
Solar evaporators Acid Plant
Driers
MgCl 2(s)
Electrolysis Unit Cl2(g)
Magnesium a) Name the three raw materials that are needed to manufacture magnesium. (3) ______b) Name the other substance produced when calcium carbonate is converted into calcium oxide. (1) ______
1 c) From the flow chart, identify two stages at which large amounts of energy are needed. (2) ______d) Write an ionic equation for the formation of magnesium hydroxide in the precipitator. (2)
e) Write a balanced chemical equation for the reaction which occurs in the neutraliser. (2)
f) State two potentially harmful leakages that this manufacturing plant could release into the local environment. (2) ______g) Name the solid product that crystallises out of the driers. (1) ______h) Suggest a site for locating this magnesium producing plant and state a reason for your choice. (2) ______
Question 2
A mining site at Broken Hill has ore containing iron and aluminium. After concentrating the ore and separating the minerals the metal ions need to be reduced. However, the methods used to reduce these metals are different. State the method used for each method and explain why these methods differ. (5) ______
Question 3 Consider the brown equilibrium mixture shown below: 2NO2(g) N2O4(g) brown colourless a) Write the expression for the equilibrium constant, Kc for this equilibrium. (1)
2 b) At 25°C, 2.43 L of an equilibrium mixture contains 0.030 moles of NO2 and 0.070 moles of N2O4. Calculate the value of Kc at 25°C. (1)
c) State the effect of increasing the total pressure at 25°C on: i) The equilibrium constant, Kc (1) ______ii) The colour of the equilibrium mixture. (1) ______
Question 4 The reaction rate of acetic acid and methanol to form methyl acetate was determined by following the moles of methyl acetate produced. CH3COOH(aq) + CH3OH(aq) CH3COOCH3(aq) + H2O(l)
Time (seconds) Moles (CH3COOCH3) 0 0.00 5 0.22 10 0.37 20 0.54 30 0.64 40 0.70 100 0.86 150 0.90 160 0.90 170 0.90 a) Draw a graph of moles versus time of methyl acetate. (4)
3 b) On the same axes, sketch how the curve would look if a suitable catalyst were added. Label this curve C. (1) c) On the same axes, sketch how the curve would look if a lower temperature were used. Label this curve T. (1) d) State and explain the function of a catalyst in a chemical reaction. (2) ______
e) Draw the energy profile diagram below to show how a catalyst affects the endothermic reaction between acetic acid and methanol. (4)
Question 5 Ammonia can be synthesised industrially by the following reaction: -1 N2(g) + 3H2(g) 2NH3(g) ΔH = -92 kJ mol a) The synthesis is carried out in the presence of finely divided iron mixed with chromium. State the effect of this mixture: i) On the rate of reaction (1) ______ii) On the equilibrium yield. (1) ______
4 Credit will be given for answers which show clear, well-expressed ideas and which present accurate and relevant information in a well organised, logical manner. Your answer should be confined to the space provided and should take approximately 10 minutes. b) In the chemical industry, Le Chatlier’s principle may be used to predict the influence of factors such as concentration, pressure and temperature on the yield of a reversible reaction. The predicted optimum conditions are sometimes impractical.
Using the reaction in the ammonia synthesis explain what is meant by the above statement. (8)
______
5 Metal Activity
Most Active Ca Al Zn Fe Least Active Cd
You may write on this section if you need more space to finish your answers. Make sure to label each answer carefully (eg Q2a continued) ______
6