Experiment 12 ( 1 / 10 )
EXPERIMENT 12
Investigating factors affecting reaction rate
Introduction Sodium thiosulphate solution and hydrochloric acid react according to the equation: 2- + S2O3 (aq) + 2H (aq) SO2(g) + S(s) + H2O(l) At first the sulphur is not visible. But as reaction goes on, more and more sulphur forms, turning the mixture white and then light yellow. The degree of cloudiness of the reaction mixture at any given moment therefore represents the extent of the reaction.
In this experiment, the reaction is carried out in a beaker placed upon a piece of white paper on which a cross has been marked. The time for the total disappearance of the cross when the contents of the beaker are viewed vertically from above is noted. This gives a measure of the time taken for a certain amount of sulphur to be formed.
A. Effect of concentration on reaction rate
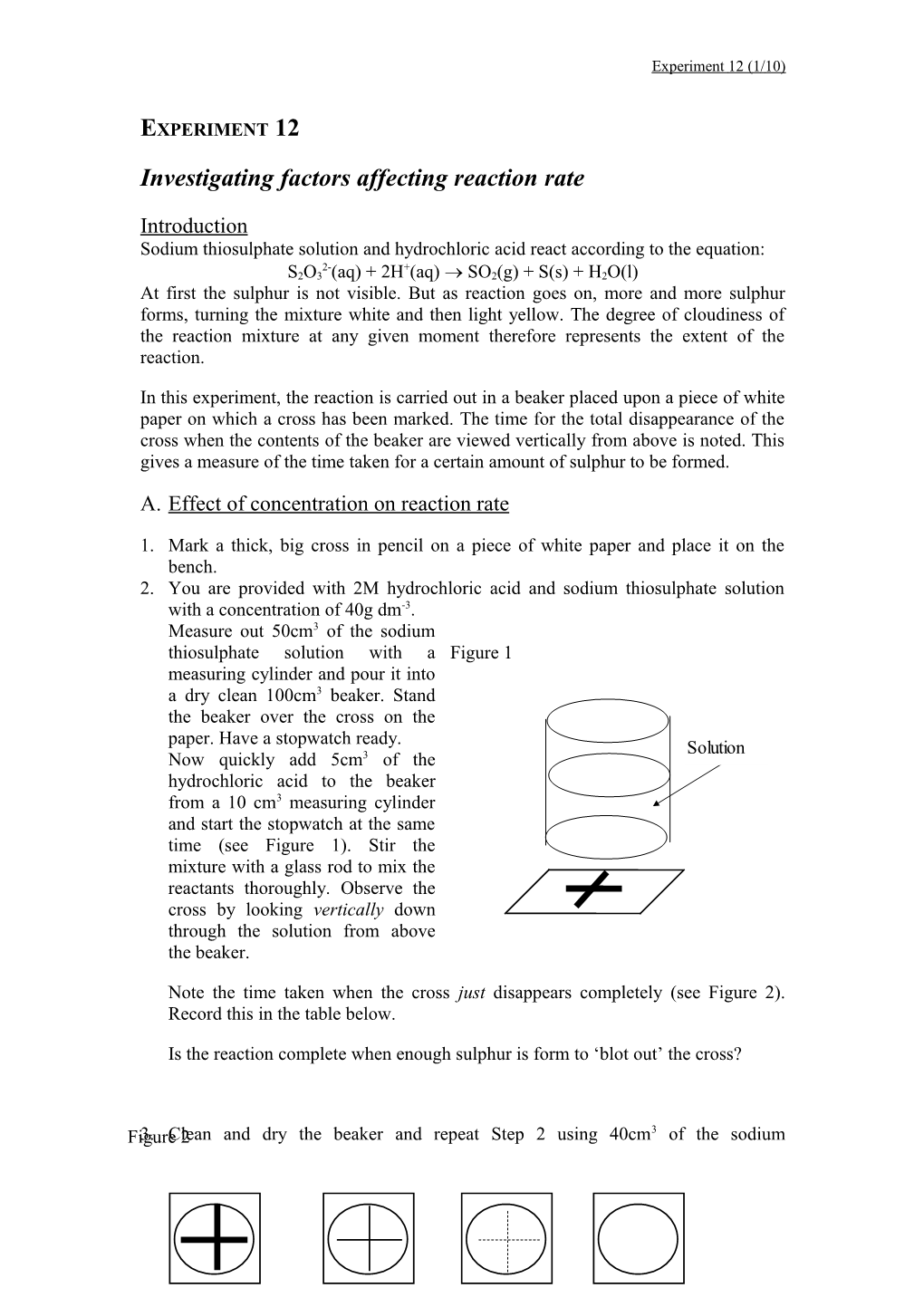
1. Mark a thick, big cross in pencil on a piece of white paper and place it on the bench. 2. You are provided with 2M hydrochloric acid and sodium thiosulphate solution with a concentration of 40g dm-3. Measure out 50cm3 of the sodium thiosulphate solution with a Figure 1 measuring cylinder and pour it into a dry clean 100cm3 beaker. Stand the beaker over the cross on the paper. Have a stopwatch ready. Solution Now quickly add 5cm3 of the hydrochloric acid to the beaker from a 10 cm3 measuring cylinder and start the stopwatch at the same time (see Figure 1). Stir the mixture with a glass rod to mix the reactants thoroughly. Observe the cross by looking vertically down through the solution from above the beaker.
Note the time taken when the cross just disappears completely (see Figure 2). Record this in the table below.
Is the reaction complete when enough sulphur is form to ‘blot out’ the cross?
Figure3. Clean 2 and dry the beaker and repeat Step 2 using 40cm3 of the sodium Experiment 12 ( 2 / 10 )
disappear completely. Repeat the experiment using the other mixtures shown in the table below. (Note that the same depth of liquid and the same marked piece of paper are used in all cases.)
Volume of Volume of Volume of Time for cross 1 Na2S2O3(aq) water HCl(aq) to disappear Time 3 3 3 (cm ) (cm ) (cm ) (s) (s-1) 50 0 5 40 10 5 30 20 5 20 30 5 10 40 5
In each case, work out the reciprocal of the time for the cross to disappear (i.e. 1/time). This is proportional to the average reaction rate during that interval of time. (Note that in each case, the amount of sulphur needed to ‘blot out’ the cross is the same). The volume of the original thiosulphate solution used in each case can be taken to be its relative concentration. Explain why this is so. Now plot a graph of 1/time against volume of the original thiosulphate solution used.
From you graph, explain how the reaction rate depends on the concentration of a reactant.
B. Effect of temperature on reaction rate
The method used here the same as that for Part A, except that in this case, temperature is the altered factor and concentrations and quantities of all reactants used are kept constant. In all cases, a reaction mixture of 10 cm3 thiosulphate solution, 40cm3 water and 5cm3 hydrochloric acid is used.
4. Mix 10cm3 of sodium thiosulphate solution with 40cm3 of water in a 100 cm3 measuring cylinder. Add the mixture to the beaker placed upon the marked paper. Quickly add 5cm3 of hydrochloric acid to the beaker and start the stopwatch at the same time. Stir the mixture with thermometer and take the temperature of the mixture. Note the time for the cross to just disappear completely. Record your results in the table below.
5. Mix another 10cm3 of thiosulphate solution with 40cm3 of water in the measuring cylinder and add the mixture to the beaker. Heat the beaker until temperature of solution is just above 35C (Figure 3). Add hydrochloric acid and repeat the rest of Step 4. (Note to record temperature after the addition of hydrochloric acid) Experiment 12 ( 3 / 10 )
Figure 3 Thermometer
5 cm3 HCl
Mixture of water and thiosulphate
Heat
1. Repeat the above procedure for temperatures of about 45C, 55C, 60C, 65C and 70C. Record your results in the table below and work out the 1/time term in each case. Temperature of reaction Time for cross to 1 mixture disappear Time (C) (s) (s-1)
Draw a graph of 1/time against temperature. Now answer the following questions. (a) How does the reaction rate change with temperature? (b) What would be the shape of the graph if the reaction rate was directly proportional to temperature? (c) Why do we use the same concentrations and quantities of reactants at the start in all cases?
(C) Effect of catalyst on rate of decomposition of hydrogen Peroxide
1. using a measuring cylinder, add 50cm3 of ‘1-volume’ hydrogen peroxide solution to a conical flask. Observe the solution carefully. Do you see any signs of decomposition? Experiment 12 ( 4 / 10 )
2. Set up the apparatus as shown in Figure 4. Make sure that the syringe reading is zero. Remove the bung. Add 0.5g of manganese (IV) oxide granules (10 - 30 mesh) to the solution and immediately replace the bung. Start the stopwatch as quickly as possible. Read the volume every 15 seconds for the first 2 minutes and every 30 seconds for the rest of the experiment, until you have obtained 2 or 3 consecutive constant readings. Before taking each reading, rotate the plunger of the syringe to ensure that it has not become jammed. Record your results in the
Figure 4
hydrogen peroxide MnO 2
table below.
3. Repeat the experiment with fresh ‘1-volume’ hydrogen peroxide solution but this time add 1.5g of the manganese (IV) oxide granules. Record your results in the table below. Time (min) 0.25 0.5 0.75 1.0 1.25 1.5 1.75
Volume of 0.5g MnO2 O2 evolved added 3 (cm ) 1.5g MnO2 added
Time (min) 2.0 2.5 3.0 3.5 4.0 4.5 5.0
Volume of 0.5g MnO2 O2 evolved added 3 (cm ) 1.5g MnO2 added
Time (min) 5.5 6.0 6.5 7.0
Volume of 0.5g MnO2 O2 evolved added 3 (cm ) 1.5g MnO2 added
Plot graphs of volume of oxygen (cm3) against time (minute) below.
Draw a tangent at each of the rate curves at time t = 0. Now answer the following questions:
1. What is the effect of manganese (IV) oxide catalyst on the rate of decomposition of hydrogen peroxide?
2. What is the effect of increased mass of manganese (IV) oxide on the (i) initial rate (ii) total time of reaction? Experiment 12 ( 5 / 10 )
3. Do you think the manganese (IV) oxide is used up in the experiments? 4. Design an experiment to test your prediction to above. Describe you method.
Class: F.6____ ( ) Name: ______Date: ______
Title: Experiment 12 – Investigating factors affecting reaction rate
Objective: To investigate: i) Effect of concentration on reaction rate ii) Effect of catalyst on reaction rate iii) Effect of temperature on reaction rate
Discussion Experiment 12 ( 6 / 10 )
A. Effect of concentration on reaction rate
Data table Volume of Volume of Volume of Time for cross 1 Na2S2O3(aq) water HCl(aq) to disappear Time 3 3 3 (cm ) (cm ) (cm ) (s) (s-1) 50 0 5 40 10 5 30 20 5 20 30 5 10 40 5
Questions and Answer
1) plot a graph of 1/time against volume of the original thiosulphate solution used.
2) Is the reaction complete when enough sulphur is form to ‘blot out’ the cross?
______
3) The reciprocal of the time for the cross to disappear (i.e. 1/time) is proportional to the average reaction rate during that interval of time.Therefore, the volume of the original thiosulphate solution used in each case can be taken to be its relative concentration. Explain why this is so.
______
______
______
______
______
4) From you graph, explain how the reaction rate depends on the concentration of a reactant.
______
______
______
B. Effect of catalyst on reaction rate
Data Table Temperature of reaction Time for cross to 1 mixture disappear Time Experiment 12 ( 7 / 10 )
(C) (s) (s-1)
Question and answer
1) Draw a graph of 1/time against temperature. 2) How does the reaction rate change with temperature?
______
______
______
3) What would be the shape of the graph if the reaction rate was directly proportional to temperature?
______
4) Why do we use the same concentrations and quantities of reactants at the start in all cases?
______
______
C. Effect of temperature on reaction rate
Data tables Time (min) 0.25 0.5 0.75 1.0 1.25 1.5 1.75
Volume of 0.5g MnO2 O2 evolved added 3 (cm ) 1.5g MnO2 added
Time (min) 2.0 2.5 3.0 3.5 4.0 4.5 5.0
Volume of 0.5g MnO2 O2 evolved added 3 (cm ) 1.5g MnO2 added Experiment 12 ( 8 / 10 )
Time (min) 5.5 6.0 6.5 7.0
Volume of 0.5g MnO2 O2 evolved added 3 (cm ) 1.5g MnO2 added
Questions and Answers
1) Plot graphs of volume of oxygen (cm3) against time (minute) below. Draw a tangent at each of the rate curves at time t = 0.
2) What is the effect of manganese (IV) oxide catalyst on the rate of decomposition of hydrogen peroxide?
______
3) What is the effect of increased mass of manganese (IV) oxide on the (i) initial rate (ii) total time of reaction? Do you think the manganese (IV) oxide is used up in the experiments?
______
______
______
4) Design an experiment to test your prediction to above. Describe you method.
______
______
______
______
______
______
Sources of errors Experiment 12 ( 9 / 10 )
Conclusion: Experiment 12 ( 10 / 10 )