Ground Water - Surface Water Interactions in the Lower Virgin River Area, Arizona and Nevada
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Yanawant: Paiute Places and Landscapes in the Arizona Strip
Yanawant Paiute Places and Landscapes in the Arizona Strip Volume Two OfOfOf The Arizona Strip Landscapes and Place Name Study Prepared by Diane Austin Erin Dean Justin Gaines December 12, 2005 Yanawant Paiute Places and Landscapes in the Arizona Strip Volume Two Of The Arizona Strip Landscapes and Place Name Study Prepared for Bureau of Land Management, Arizona Strip Field Office St. George, Utah Prepared by: Diane Austin Erin Dean Justin Gaines Report of work carried out under contract number #AAA000011TOAAF030023 2 Table of Contents Preface……………………………………………………………………………………………ii i Chapter One: Southern Paiute History on the Arizona Strip………………………………...1 Introduction.............................................................................................................................. 1 1.1 Early Southern Paiute Contact with Europeans and Euroamericans ........................... 5 1.2 Southern Paiutes and Mormons ........................................................................................ 8 1.3 The Second Powell Expedition......................................................................................... 13 1.4 An Onslaught of Cattle and Further Mormon Expansion............................................ 16 1.5 Interactions in the First Half of the 20 th Century ......................................................... 26 Chapter Two: Southern Paiute Place Names On and Near the Arizona Strip 37 Introduction ........................................................................................................................... -

TMDL Water Quality Study of the Virgin River Watershed
TMDL Water Quality Study of the Virgin River Watershed DRAFT January 28, 2004 Submitted to: Utah Department of Environmental Quality Division of Water Quality 288 North 1460 West Salt Lake City, UT 84116 Kent Montague Project Manager Harry Lewis Judd Project Supervisor Submitted by: Tetra Tech, Inc. Water Resources and TMDL Center Utah Division of Water Quality TMDL Water Quality Study of the Virgin River CONTENTS 1.0 Introduction.......................................................................................................................................1 2.0 Water Quality Standards ...................................................................................................................5 2.1 303(d) List Status ........................................................................................................................5 2.2 Parameters of Concern.................................................................................................................7 2.2.1 Salinity and Total Dissolved Solids ........................................................................................7 2.2.2 Temperature ............................................................................................................................8 2.2.3 Total Phosphorus and Dissolved Oxygen ...............................................................................8 2.2.4 Selenium..................................................................................................................................8 2.3 Applicable -

Beaver DAM Wash National Conservation
Beaver Dam Wash BLM National Conservation Area What is a National Conservation Area? Through the Omnibus Public Land Management Act of 2009, Congress designated the Beaver Dam Wash National Conservation Area (NCA) in Washington County, Utah “to conserve, protect, and enhance… the ecological, scenic, wildlife, recreational, cultural, historical, natural, educational, and scientific resources” of these public lands. The new NCA comprising roughly of 63,500 acres is managed by the BLM’s St. George Field Office. Where is the Beaver Dam Wash NCA? Located in the southwest corner of Washington County, Utah, the Beaver Dam Wash NCA is approximately 22 miles west of St. George, Utah and roughly 13 miles northwest of Littlefield, Arizona. The Nevada and Arizona state lines, border the western section of the NCA and Interstate 15 and the Virgin River parallel its southern boundary. U.S. Highway 91 is the only paved highway through the NCA. What Are the Special Values of the Beaver Dam Wash NCA? This NCA is within an ecological transition zone between the Mojave Desert and the Great Basin. Creosote bush, white bursage, and other desert shrubs grow at lower elevations and provide habitat for desert bighorn sheep and the Mojave Desert tortoise, a threatened species listed under the Endangered Species Act. Joshua trees and dense stands of blackbrush cover St. George Field Office the slopes of the Beaver Dam Mountains, which rise along the eastern boundary of the NCA. Surface water flows in the upper reaches of Beaver Dam Wash, but rarely travels all the way through the NCA. Riparian vegetation along the stream channel is important habitat for migratory birds and other wildlife. -
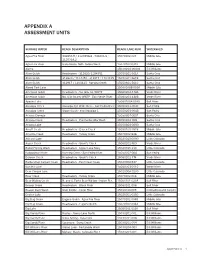
Appendix a Assessment Units
APPENDIX A ASSESSMENT UNITS SURFACE WATER REACH DESCRIPTION REACH/LAKE NUM WATERSHED Agua Fria River 341853.9 / 1120358.6 - 341804.8 / 15070102-023 Middle Gila 1120319.2 Agua Fria River State Route 169 - Yarber Wash 15070102-031B Middle Gila Alamo 15030204-0040A Bill Williams Alum Gulch Headwaters - 312820/1104351 15050301-561A Santa Cruz Alum Gulch 312820 / 1104351 - 312917 / 1104425 15050301-561B Santa Cruz Alum Gulch 312917 / 1104425 - Sonoita Creek 15050301-561C Santa Cruz Alvord Park Lake 15060106B-0050 Middle Gila American Gulch Headwaters - No. Gila Co. WWTP 15060203-448A Verde River American Gulch No. Gila County WWTP - East Verde River 15060203-448B Verde River Apache Lake 15060106A-0070 Salt River Aravaipa Creek Aravaipa Cyn Wilderness - San Pedro River 15050203-004C San Pedro Aravaipa Creek Stowe Gulch - end Aravaipa C 15050203-004B San Pedro Arivaca Cienega 15050304-0001 Santa Cruz Arivaca Creek Headwaters - Puertocito/Alta Wash 15050304-008 Santa Cruz Arivaca Lake 15050304-0080 Santa Cruz Arnett Creek Headwaters - Queen Creek 15050100-1818 Middle Gila Arrastra Creek Headwaters - Turkey Creek 15070102-848 Middle Gila Ashurst Lake 15020015-0090 Little Colorado Aspen Creek Headwaters - Granite Creek 15060202-769 Verde River Babbit Spring Wash Headwaters - Upper Lake Mary 15020015-210 Little Colorado Babocomari River Banning Creek - San Pedro River 15050202-004 San Pedro Bannon Creek Headwaters - Granite Creek 15060202-774 Verde River Barbershop Canyon Creek Headwaters - East Clear Creek 15020008-537 Little Colorado Bartlett Lake 15060203-0110 Verde River Bear Canyon Lake 15020008-0130 Little Colorado Bear Creek Headwaters - Turkey Creek 15070102-046 Middle Gila Bear Wallow Creek N. and S. Forks Bear Wallow - Indian Res. -

Geophysical Constraints on the Virgin River Depression, Nevada, Utah, and Arizona by V.E
U.S. DEPARTMENT OF THE INTERIOR U.S. GEOLOGICAL SURVEY Geophysical Constraints on the Virgin River Depression, Nevada, Utah, and Arizona by V.E. Langenheim1 , J.M. Glen1 , R.C. Jachens1 , G.L. Dixon2 , T.C. Katzer3 , and R.L. Morin1 Open-File Report 00-407 2000 This report is preliminary and has not been reviewed for conformity with U.S. Geological Survey editorial standards or with the North American Stratigraphic Code. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. U.S. DEPARTMENT OF THE INTERIOR U.S. GEOLOGICAL SURVEY 1Menlo Park, California 2Las Vegas, Nevada 3Cordilleran Hydrology, Inc., Reno, Nevada TABLE OF CONTENTS Abstract.................................................................................................................... 1 Introduction.............................................................................................................. 1 Geologic Setting....................................................................................................... 1 Previous Geophysical Work .................................................................................... 2 Drill-Hole Data and Physical Properties .................................................................. 3 Gravity and Magnetic Data....................................................................................... 4 Depth to Basement................................................................................................... 7 Method................................................................................................................. -

VVHS 100 Year History.Pdf
A HISTO Y OF EDUCATION IN THE VIRGIN VALLEY TABLE OF CONTENTS Introduction Page 1 Chapter First schools to 1890 Page 3 Chapter 2 1890 to 1905 Page 8 Chapter 3 1905 to 1920 Page 12 Chapter 4 1920 to 1930 Page 17 Chapter 5 1930 to 1940 Page 21 Chapter 6 1940 to 1950 Page 28 Chapter 7 1950 to 1970 Page 40 Chapter 8 1970 to Present Page 46 Credits Page 57 Introduction This book was undertaken as a English IV Distinguished Scholar research project. It endeavors to cover the history of education from the first settlement in 1877 in Bunkerv ll 1 e to the present day. Th 1 s year was chosen for the project to mark the last year the students of the Virgin Valley High School would spend on the present campus and to commemorate the move to the new facility. Many people in the Virgin Valley have contributed information and spent hours helping the seniors research this book. Throughout the text, the memor l es and research of Vinnie Leavitt and DeMar Hughes are used as the basis for the history. Others who have contributed are E. Wesley Hughes, Marion ~Tex~ Hughes, Gertha Norvell, Myrtle Reber, Rene Adams, Emmett Barnum, Don Lee, Jack and Vi.late Hardy, Larry and Colleen Reber, Mike Waite, Evan Wilson, Kirk Brotherson, Joe Bowler, Kent Anderson and Harold Wittwer. For their help and encouragement, we are extremely grateful. A bibliography of the articles and histories which were used in each chapter are listed at the end. As the research was gathered, there were discrepencies 1 n some of the art i c I es. -

Kane County, Utah Resource Management Plan
Kane County Resource Management Plan Adopted 28 November 2011 KANE COUNTY, UTAH RESOURCE MANAGEMENT PLAN For the Physical Development of the Unincorporated Area Pursuant to Section 17-27-301 of the Utah Code ADOPTED 28 NOVEMBER 2011 Should any part of the Kane County Resource Management Plan be determined invalid, no longer applicable or need modification, those changes shall affect only those parts of the Plan that are deleted, invalidated or modified and shall have no effect on the remainder of the Resource Management Plan. This document was prepared by the Division of Community and Economic Development of the Five County Association of Governments under the guidance and direction of the Kane County Resource Development Committee, Kane County Land Use Authority and the Board of County Commissioners. Funding used to prepare this document came from Kane County contributions, a Regional Planning grant from the Utah Permanent Community Impact Board and a Planning and Technical Assistance Grant from the U.S. Department of Commerce, Economic Development Administration. - 1 - Kane County Resource Management Plan Adopted 28 November 2011 Acknowledgments Every effective planning process includes a multitude of individuals if it is to be successful. This effort is no different. Many individuals have had an impact upon the preparation and adoption of this Plan. However, most important are the residents of Kane County, who have responded to surveys, interviews, and attended public meetings and hearings. All who did so should be commended for their desire to be a participant in determining the future of Kane County. Some specific individuals and groups have had intensive involvement in the Kane County planning process, and are acknowledged below: Kane County Commission Kane County Land Use Authority Doug Heaton, Chairman Shannon McBride, Land Use Administrator Dirk Clayson Tony Chelewski, Chairman Jim Matson Roger Chamberlain Wade Heaton Kane County Staff Robert Houston Verjean Caruso, Co. -

Preliminary Geologic Map of the Las Vegas I°X2° Quadrangle, Nevada
UNITED STATES DEPARTMENT OF THE INTERIOR GEOLOGICAL SURVEY Preliminary Geologic Map of the Las Vegas I°x2° quadrangle, Nevada, Arizona and California Compiled by Robert G. Bohannon Open-File Report 78-670 -f. 1978 This report is preliminary and has not been edited or reviewed for conformity with U.S. Geological Survey standards and nomenclature. V'OKK:-::.AT IO.N c:- ? UNITS [^»1 VP Ploiiitoc:one{?) QUATERNARY Ql and Pleistocene Cm Qrci SOUTH QUATERNARY AND QTal V^ESTERN EASTERN TERTIARY AREA AREA Pliocene and Tra _. Tb 1 Ipli i! !(Mir Miocene Ths Tppv Thv Thl Tmdvl STrvi 1 Thb Miocene TERTIARY Tht Tsu -\ Upper Kb Cretaceous Lower - CRETACEOUS Kwt Cretaceous J-Ra " JURASSIC TRIASSIC(?) "Bine I Middle (?) and h TRIASSIC *« *\ Lower Triassic< Pkt PERMIAN PC P1M P ENXS YLV AN I AN MISSISSIPPIAN Mni Dms Dmp jFzuj DEVONIAN SI SIL'JRIAN -C-D ORDOVICIAN ^^ Cdl -Cu pCsj - PRECAM8RIAN I pCgn pCrg INSCRIPTION OF MAP UNITS Qal ALLUVIAL DEPOSITS (KOLOCENE AND PLEISTOCENE?) Various types of young alluvial deposits; mostly dissected alluvial cover, terrace deposits, and channel fill; also includes sand dunes, talus, gypsiferous terrace deposits, and tufa. Predominately unconsolidated, caliche horizons common Qp PLAYA DEPOSITS (HOLOCENE AND PLEISTOCENE?) Fine-grained, unconsolidated to poorly consolidated sand, silt, and clay; locally interfinger with alluvial deposits Qc CHEMEHUEVI FORMATION (PLEISTOCENE) Light-colored poorly consolidated sand, silt, and clay. Probably depos ited in large lake that once covered area of Lake Mead and Colorado River Ql LAS VEGAS FORMATION (PLEISTOCENE) Light-colored, poorly consolidated clay and silt; abundant mollusk frag ments and some vertebrate remains occur. -

Municipal Deliveries of Colorado River Basin Water
Municipal Deliveries of Colorado River Basin Water Author Michael J. Cohen Research Assistant Jenifer C. Martin Editors Nancy Ross Paula Luu Pacific Institute 654 13th Street, Preservation Park Oakland, California 94612 www.pacinst.org Phone: 510.251.1600 Facsimile: 510.251.2203 © Copyright 2011. All Rights Reserved ISBN: 1-893790-34-7 ISBN 13: 978-1-893790-34-6 Cover Photo: Aerial view of the Whitsett Pumping Plant, Courtesy © The Metropolitan Water District of Southern California Note – this 8/21/2011 revision corrects: population data for the City of Westminster, water delivery data for Denver Water, GPCD calculations for Grand Valley, CO (Grand Junction), and 2005 Colorado River deliveries for San Luís Río Colorado, Mexico. Municipal Deliveries of Colorado River Basin Water About the Pacific Institute The Pacific Institute is one of the world’s leading independent nonprofits conducting research and education to create a healthier planet and sustainable communities. Based in Oakland, California, with an office in Boulder, Colorado, we conduct interdisciplinary research and partner with stakeholders to produce solutions that advance environmental protection, economic development, and social equity—in California, nationally, and internationally. We work to change policy and find real-world solutions to problems like water shortages, habitat destruction, global warming, and environmental injustice. Since our founding in 1987, the Pacific Institute has become a locus for independent, innovative thinking that cuts across traditional areas of study, helping us make connections and bring opposing groups together. The result is effective, actionable solutions addressing issues in the fields of freshwater resources, climate change, environmental justice, and globalization. More information about the Institute and our staff, directors, funders, and programs can be found at www.pacinst.org. -

Grand Canyon Council Oa Where to Go Camping Guide
GRAND CANYON COUNCIL OA WHERE TO GO CAMPING GUIDE GRAND CANYON COUNCIL, BSA OA WHERE TO GO CAMPING GUIDE Table of Contents Introduction to The Order of the Arrow ....................................................................... 1 Wipala Wiki, The Man .................................................................................................. 1 General Information ...................................................................................................... 3 Desert Survival Safety Tips ........................................................................................... 4 Further Information ....................................................................................................... 4 Contact Agencies and Organizations ............................................................................. 5 National Forests ............................................................................................................. 5 U. S. Department Of The Interior - Bureau Of Land Management ................................ 7 Maricopa County Parks And Recreation System: .......................................................... 8 Arizona State Parks: .................................................................................................... 10 National Parks & National Monuments: ...................................................................... 11 Tribal Jurisdictions: ..................................................................................................... 13 On the Road: National -

Virgin Valley Water District
Virgin Valley Water District • About the District • Where Our Water Comes From • About the System About the District - History • 1800’s water was pulled directly from the Virgin River, mixed with ashes, settled in cisterns and used for water • First well drilled in 1930 • 1933 - first town piping installed near Bunkerville school that also served 10 homes • Mid 1930’s springs on Virgin Mountain developed and piped to both Bunkerville and Mesquite Cabin Canyon Spring About the District - History Bunkerville Park Well (Well 29 Site) • 1940’s Bunkerville Water User’s and Mesquite Farmstead Water Associations were created and started supplying water • Virgin Valley Water District Created in 1993 by State Legislature – Combined assets of the two water companies • Hyper growth in late 90’s to 2008 • Arsenic treatment plants installed in 2009 • Current operations Groundwater Basin 222 Shared between Nevada, Utah, and Arizona Groundwater Basin 222 – Where VVWD Gets it’s Groundwater From Colorado River Water Basin The Colorado River Water Shed Virgin River Water Basin The Virgin River Arm of the Colorado River Watershed About the System VVWD Service Boundary and Springs Location About the System Major Transmission Lines: -Black 24” -Burnt Orange 18” -Yellow 16” -Purple 14” -Blue 12” About the System System Pressure Zone and Well Feeding Map Consultants, Vendors & Contractors The Cost of Providing Water to the Public What the Public Expects: Maintenance and Repair: Things Eventually Wear Out Pipe, Booster Pumps, Meter Vaults, Wells, Valves, Hydrants, -

Mineral Resources of the Clover Mountains Wilderness Study Area, Lincoln County, Nevada
Mineral Resources of the Clover Mountains Wilderness Study Area, Lincoln County, Nevada U.S. GEOLOGICAL SURVEY BULLETIN 1729-D t **. i &. i-wj'-sA" ..,. r .»\ '* ~ "'" ^^-J5?#^ Chapter D Mineral Resources of the Clover Mountains Wilderness Study Area, Lincoln County, Nevada By BARRY MORING, H. RICHARD BLANK, Jr., and JAMES D. HOFFMAN U.S. Geological Survey EDWARD L McHUGH U.S. Bureau of Mines U.S. GEOLOGICAL SURVEY BULLETIN 1729 MINERAL RESOURCES OF WILDERNESS STUDY AREAS: SOUTHEASTERN NEVADA DEPARTMENT OF THE INTERIOR DONALD PAUL MODEL, Secretary U.S. GEOLOGICAL SURVEY Dallas L. Peck, Director Any use of trade names and trademarks in this publication is for descriptive purposes only and does not constitute endorsement by the U.S. Geological Survey UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1988 For sale by the Books and Open-File Reports Section U.S. Geological Survey Federal Center, Box 25425 Denver, CO 80225 Library of Congress Cataloging-in-Publication Data Mineral resources of the Clover Mountains Wilderness Study Area, Lincoln County, Nevada. (U.S. Geological Survey bulletin ; 1729-D) Bibliography: p. 1. Mines and mineral resources Nevada Clover Mountains Wilderness. 2. Clover Mountains Wilderness (Nev.) I. Moring, Barry, C. II. Series. QE75.B9 no. 1729-D 557.3 s 88-600195 [TN24.N3] [553'.09793'14] STUDIES RELATED TO WILDERNESS Bureau of Land Management Wilderness Study Area The Federal Land Policy and Management Act (Public Law 94-579, October 21, 1976) requires the U.S. Geological Survey and the U.S. Bureau of Mines to conduct mineral surveys on certain areas to determine the mineral values, if any, that may be present.