ME 354 Tutorial, Week #9 Jet-Propulsion Cycle A turbojet is flying with a velocity of 320 m/s at an altitude of 9150m, where the ambient conditions are 32 kPa and -32C. The pressure ratio across the compressor is 12, and the temperature at the turbine inlet is 1400 K. Air enters the compressor at a rate of 40 kg/s, and the jet fuel has a heating value of 42,700 kJ/kg. Assuming ideal operation for all components and constant specific heats for air at room temperature, determine:
a) the temperature and pressure at the turbine exit, b) the velocity of the exhaust gases, c) the propulsive power developed, d) the propulsive efficiency, and e) the rate of fuel consumption.
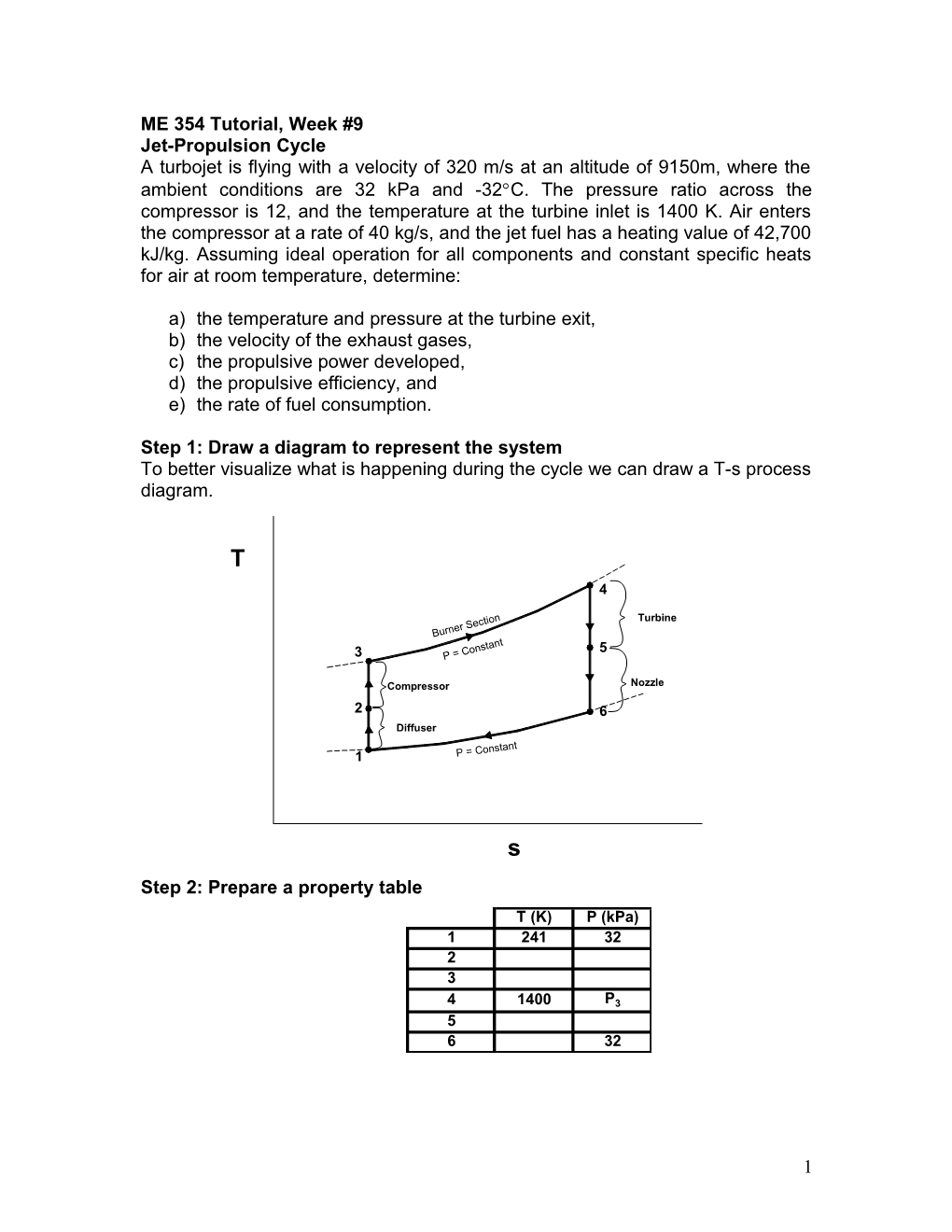
Step 1: Draw a diagram to represent the system To better visualize what is happening during the cycle we can draw a T-s process diagram.
T 4
Turbine
3 5
Compressor Nozzle 2 6 Diffuser
1
s Step 2: Prepare a property table T (K) P (kPa) 1 241 32 2 3
4 1400 P3 5 6 32
1 Step 3: State your assumptions Assumptions:
1) pe 0 for all components
2) ke 0 for compressor, burner, and turbine sections ONLY 3) air-standard assumptions are applicable 4) air is an ideal gas with constant specific heats at room temperature 5) ideal & steady operation of all components 6) wcomp,in = wturb,out
Step 4: Calculations Part a) In order to determine the temperature at the turbine exit we can start by writing an energy balance over the turbine as shown in Eq1. Note: we have applied the steady operating conditions assumption and the assumption that ke & pe 0 in the energy balances of Eq1 and Eq3.
wturb,out (h4 h5 ) (Eq1)
Since we have modeled the air as an ideal gas with constant specific heats at room temperature [Recall: h=h(T)] we can rewrite Eq1 as Eq2.
wturb,out (h4 h5 ) c p (T4 T5 ) (Eq2)
Writing an energy balance for the compressor, we obtain Eq3.
wcomp,in (h3 h2 ) c p (T3 T2 ) (Eq3)
Since we have assumed the work produced by the turbine is equal to the work supplied to the compressor we can equate Eq2 to Eq3, to obtain Eq4.
wturb,out wcomp,in c p (T4 T5 ) c p (T3 T2 ) (Eq4)
Isolating for T5 in Eq4 we obtain Eq5.
T5 T4 T2 T3 (Eq5)
Since we are given T4 in the problem statement, we must solve for T2 and T3 in order to find T5. Performing an energy balance on the diffuser, we obtain Eq6.
d E cv m air ke pe u Pv ke pe u Pv (Eq6) dt 1 2
2 Using the steady operation assumption, the assumption that pe 0, and the definition of enthalpy (h = u + Pv), Eq6 reduces to Eq7. Since we are analyzing a diffuser we can assume V2 0 (of negligible velocity), therefore ke2 0.
2 2 V1 V1 ke1 h1 h2 0 c p (T2 T1 ) T2 T1 (Eq7) 2 2c p
Assuming we are flying in still air, the velocity of the air (relative to the jet) at the inlet of the diffuser is equal to the velocity of the jet. Therefore, V 1 = 320 m/s. From Table A-2 for air at room temperature cp = 1.005 kJ/kg*K. Substituting these values into Eq7 we can solve for the temperature at location 2.
2 2 2 m m 320 2 102400 2 V 2 s s T 1 T 241 K 241 K 2 1 2 2c p kJ 3 m 21.005 2.0110 2 kg K s K
T2 = 291.9 K
We can find the pressure at location 2 by noting that the diffuser process is modeled as isentropic. We can use the ideal gas relation for isentropic processes as shown below. From Table A-2 for air at room temperature k = 1.4.
k 1 k k 1.4 T P k P T k 1 T k 1 291.9K 0.4 2 2 2 2 2 P2 P1 (32kPa) T1 P1 P1 T1 T1 241K
P2 = 62.6 kPa
Using the compressor’s pressure ratio of 12 (given in the problem statement), we can solve for the pressure at location 3.
P3 12P2 12(62.6kPa)
P3 = 751.2 kPa
Since the compression process is modeled as isentropic, we can solve for the temperature at the compressor exit, T3, using the ideal gas relation for isentropic processes as shown below.
3 k 1 k 1 k k 0.4 T3 P3 P3 1.4 T3 T2 (291.9K)12 T2 P2 P2
T3 =593.7 K
Having determined T2 & T3, we can use Eq5 to determine the temperature at the turbine exit.
T5 T4 T2 T3 1400[K] 291.9[K] 593.7[K]
T5 =1098.2 K Answer a)
We can find the pressure at the turbine exit by noting the expansion process through the turbine is modeled as isentropic. We can use the ideal gas relation for isentropic processes as shown in Eq8.
k 1 k k T P k P T k1 T k 1 5 5 5 5 5 (Eq8) P5 P4 T4 P4 P4 T4 T4
The pressure across the burner section (3 4) is constant so P4 = P3 = 751.2 kPa. We are given T4 in the problem statement and we determined T5 in the previous step. Substituting these values into Eq8, we can solve for P5.
1.4 1098.2[K] 0.4 P5 (751.2[kPa]) 1400[K]
P5 =321.1 kPa Answer a)
Part b) To find the velocity of the exhaust gases we must first perform an energy balance on the nozzle (5 6) as shown in Eq9.
d E cv m air ke pe u Pv ke pe u Pv (Eq9) dt 5 6
Using the steady operation assumption, the assumption that pe 0, and the definition of enthalpy (h = u + Pv), Eq9 reduces to Eq10. Since we are analyzing a nozzle we can assume V5 0 (of negligible velocity), therefore ke5 is also zero.
4 V 2 h ke h 0 6 c (T T ) V 2c (T T ) (Eq10) 5 6 6 2 p 5 6 6 p 5 6
From Eq10, we see we must first determine the temperature at the exit of the nozzle (Location 6).
Since the process through the nozzle is modeled as isentropic, we can use the ideal gas relation for isentropic processes as shown below. The pressure at location 6 is ambient (32 kPa).
k1 k1 0.4 T P k P k 32[kPa] 1.4 6 6 6 T6 T5 (1098.2K) T5 P5 P5 321.1[kPa]
T6 = 568.2 K
Substituting this result into Eq10 we can solve for the velocity of the exhaust gases.
J m2 V 21.005(103 ) 1098.2[K] 568.2[K] 1065300 6 2 kg K s
V6 = 1032 m/s Answer b)
Part c) The propulsive power developed is defined as shown in Eq11.
(Eq11) W p mair (Vexit Vinlet )Vaircraft
Substituting in the known values into Eq11, we can solve for the propulsive power developed (it was previously noted that Vinlet = Vaircraft).
kg m m m kg m m W p 40 1032 320 320 9113600 2 s s s s s s
W p 9113.6 kW Answer c)
Part d) The propulsive efficiency is defined as the propulsive power developed divided by the rate of heat input as shown in Eq12.
5 W p p (Eq12)
Qin
We determined the propulsive power developed in part c). The rate of heat input can be determined from an energy balance on the burner section (3 4) as shown in Eq13.
Qin mair h4 h3 mair c p T4 T3 (Eq13)
Substituting in the known values into Eq13 we can solve for the rate of heat input.
kg kJ Q 40 1.005 1400[K] 593.7[K] in s kg K
32413.3 kW Qin
Substituting in the known values into Eq12 we can solve for propulsive efficiency.
W p 9113.6[kW ] p =0.2812 or 28.12% Answer d) 32413.3[kW ] Qin
Part e) The rate of fuel consumption can be determined from the heating value of fuel and the rate of heat input as shown in Eq14.
kJ 32413.3 Qin s m fuel 0.759 kg/s Answer e) HV kJ 42700 kg
Step 5: Concluding Remarks & Discussion a) The temperature and pressure at the turbine exit are 1098.2 K & 321.1 kPa respectively. b) The exhaust gas velocity is 1032 m/s. c) The propulsive power developed is 9113.6 kW. d) The propulsive efficiency is 28.12% e) The rate of fuel consumption is 0.759 kg/s.
6