More SN1 and SN2!
1. Which of the following alkyl halides undergoes the fastest SN2 reaction with sodium cyanide, NaCN? Draw the product. a. bromomethane(CH3CN) b. tert-butyl bromide c. 2-bromopropane d. bromoethane
2. In which of the following solvents would the reaction of 1-bromobutane with sodium chloride, NaCl, proceed the fastest? a. acetic acid b. acetonitrile (aprotic, polar – SN2) c. water d. methanol
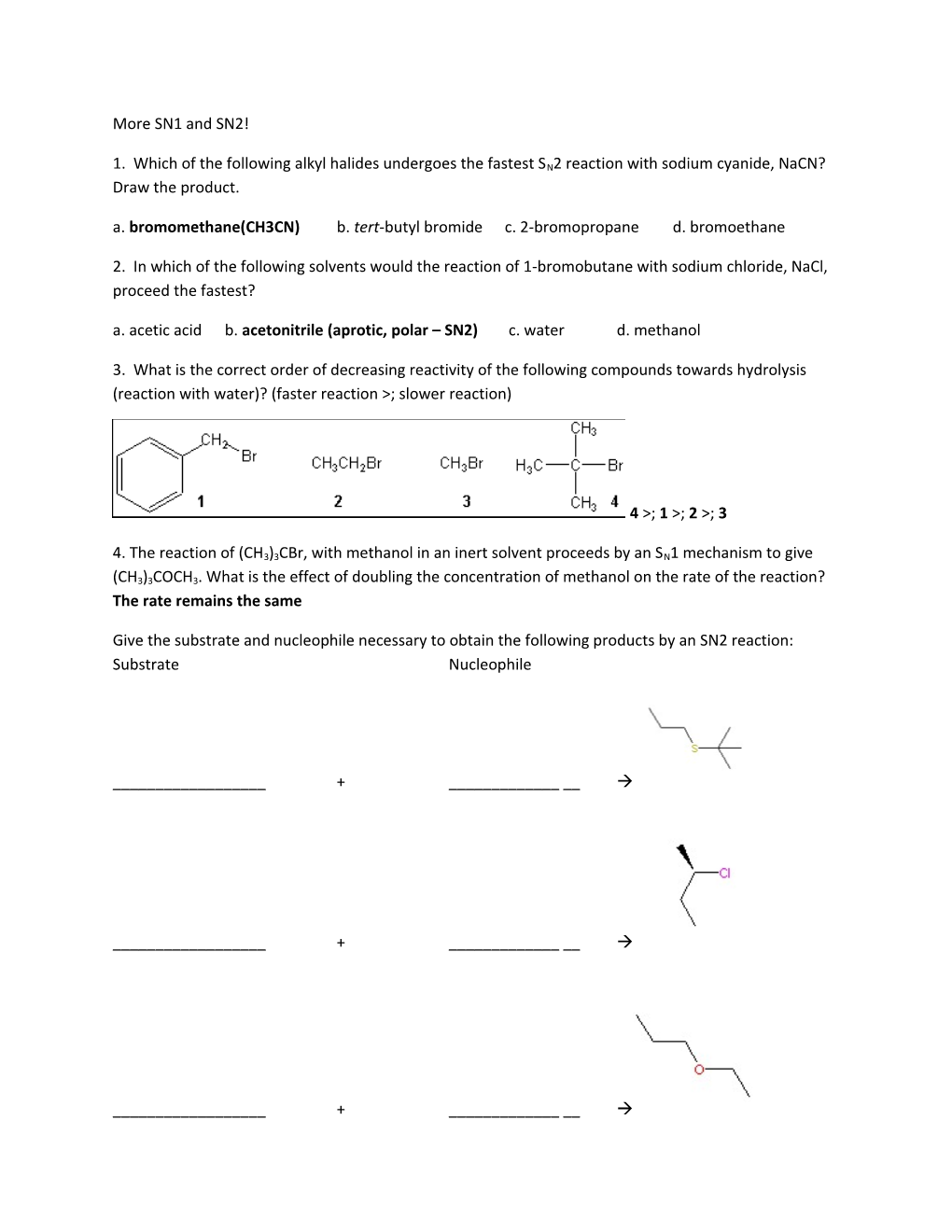
3. What is the correct order of decreasing reactivity of the following compounds towards hydrolysis (reaction with water)? (faster reaction >; slower reaction)
4 >; 1 >; 2 >; 3
4. The reaction of (CH3)3CBr, with methanol in an inert solvent proceeds by an SN1 mechanism to give
(CH3)3COCH3. What is the effect of doubling the concentration of methanol on the rate of the reaction? The rate remains the same
Give the substrate and nucleophile necessary to obtain the following products by an SN2 reaction: Substrate Nucleophile
______+ ______
______+ ______
______+ ______ Draw the transition state and product for the following reaction:
+ OH-
Write a reasonable mechanism for the following reaction:
------CH3CH2OH--->