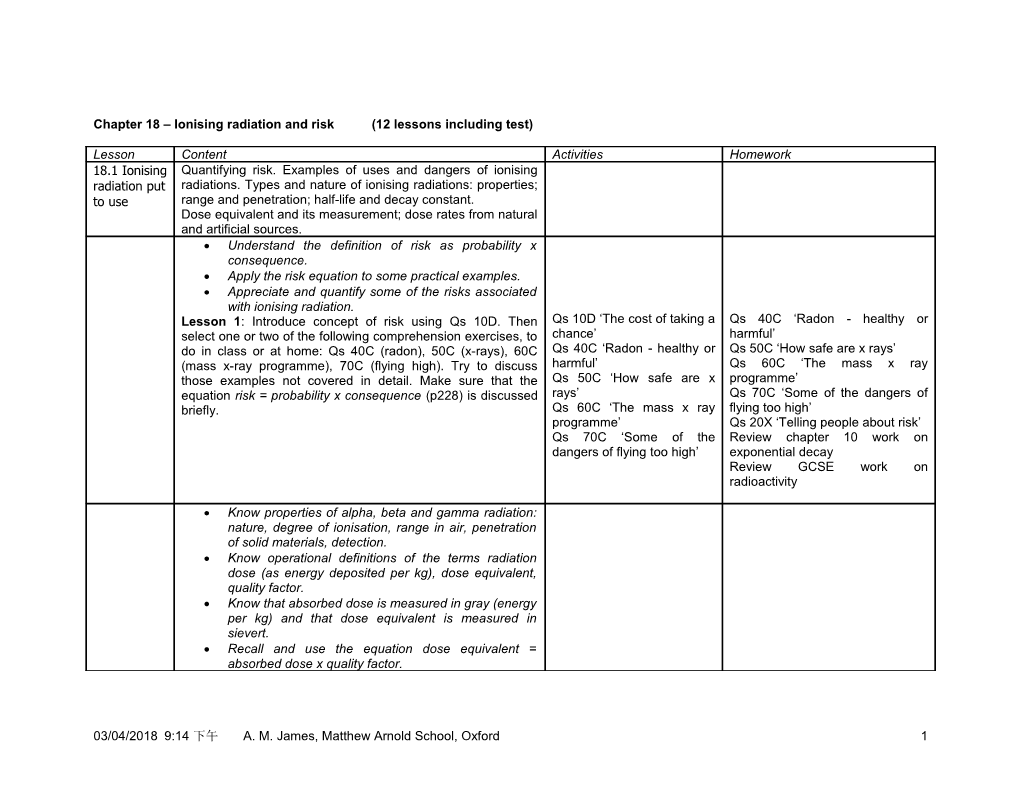
Chapter 18 – Ionising radiation and risk (12 lessons including test)
Lesson Content Activities Homework 18.1 Ionising Quantifying risk. Examples of uses and dangers of ionising radiation put radiations. Types and nature of ionising radiations: properties; to use range and penetration; half-life and decay constant. Dose equivalent and its measurement; dose rates from natural and artificial sources. Understand the definition of risk as probability x consequence. Apply the risk equation to some practical examples. Appreciate and quantify some of the risks associated with ionising radiation. Lesson 1: Introduce concept of risk using Qs 10D. Then Qs 10D ‘The cost of taking a Qs 40C ‘Radon - healthy or select one or two of the following comprehension exercises, to chance’ harmful’ do in class or at home: Qs 40C (radon), 50C (x-rays), 60C Qs 40C ‘Radon - healthy or Qs 50C ‘How safe are x rays’ (mass x-ray programme), 70C (flying high). Try to discuss harmful’ Qs 60C ‘The mass x ray those examples not covered in detail. Make sure that the Qs 50C ‘How safe are x programme’ equation risk = probability x consequence (p228) is discussed rays’ Qs 70C ‘Some of the dangers of briefly. Qs 60C ‘The mass x ray flying too high’ programme’ Qs 20X ‘Telling people about risk’ Qs 70C ‘Some of the Review chapter 10 work on dangers of flying too high’ exponential decay Review GCSE work on radioactivity
Know properties of alpha, beta and gamma radiation: nature, degree of ionisation, range in air, penetration of solid materials, detection. Know operational definitions of the terms radiation dose (as energy deposited per kg), dose equivalent, quality factor. Know that absorbed dose is measured in gray (energy per kg) and that dose equivalent is measured in sievert. Recall and use the equation dose equivalent = absorbed dose x quality factor.
03/04/2018 9:14 下午 A. M. James, Matthew Arnold School, Oxford 1 Lesson Content Activities Homework Know some of the contributions to annual exposure to ionising radiation, such as cosmic rays, food etc. Use given dose equivalent (Sv) and risk information (% risk of developing cancer) to calculate risk for particular situations Lesson 2: Recap properties of alpha, beta and gamma Demonstrate alpha, beta Some of: radiation. and gamma sources. Qs 40C ‘Radon - healthy or Discuss radiation shielding (qualitatively), radiation dose harmful’ measurements, quality factors, dose equivalents and units, in FPP Jan. 08 Q12; June 07 Qs 50C ‘How safe are x rays’ context of comprehension problems and experiments to be Q9 Qs 60C ‘The mass x ray attempted in subsequent lessons. Include sample calculations, programme’ and discuss relative contributions to body dose equivalent Qs 70C ‘Some of the dangers of from various background sources. flying too high’ Qs student book p229
Know properties of alpha, beta and gamma radiation: nature, degree of ionisation, range in air, penetration of solid materials. Observe ionisation by alpha and beta radiation using spark chamber and/or cloud chamber.
Recall and use the equation I = I0exp (-x), including logarithmic forms, to perform calculations on the shielding properties of various materials, including determination of half-thickness. Observe absorption of ionising radiation by biological materials. Observe rapid decay/short half-life of Pa-234. Lesson 3-4: If time is pressing, the work of these two lessons could be curtailed and done in one lesson. Activity 30E ‘Radiation all Some of: Do Activity 30E with data logging kit. around’ Qs 80C ‘Hot news: a radioactive If not done so already, next use GM tube detector with alpha Activity 50E ‘Rays make leak in Japan’ and/or beta sources to show range and penetrating properties ions’ Qs 90C ‘Industrial radiography’ (Activities 70E, 90E). Compare with gamma source and note Activity 90E ‘Range of beta Qs 100C ‘Smoke detector’ the existence of background radiation. particles in aluminium and Qs 110C ‘Radioisotope tracers in Discuss shielding calculations (p224), giving students data on lead’ the oil industry’ aluminium to determine its absorption half-thickness, using the Activity 100E ‘Absorption in Qs 130C ‘Radiation protection and
03/04/2018 9:14 下午 A. M. James, Matthew Arnold School, Oxford 2 Lesson Content Activities Homework
equation I = I0exp (-x) (See file “rad info”). biological materials’ dosimetry’ Having covered the idea of absorption, do A100E using a Qs 160C ‘Radiation processing’ variety of biological absorbers (fruit good here). Homework FPP June 08 Q8; G495 Qs 180C ‘Teletherapy : treatment while these lessons are taking place should include various Specimen Q10; June 08 Q5; of tumours by ionising radiation’ comprehension questions (Qs 80C-180C). Jan. 07 Q9(c) Determine absorption half- thickness for aluminium Investigate radioactive decay using software modelling activity. Recall and use the equation number of moles = mass / molar mass when performing calculations on radioactive decay processes.
Use the equations A = -N, N = N0exp (-t), t1/2 = ln2/ to perform calculations on radioactive decay processes. Lesson 5: Recap chapter 10 work on exponential nature of Activity 130S ‘Exploring Activity 130S ‘Exploring radioactive decay’ radioactive decay’ radioactive decay. Make sure that t1/2 = 0.693/ and A = N are included, with sample calculations. Qs 120S ‘Summary questions for Carry out software Activity 130S ‘Exploring radioactive decay’. FPP G495 Specimen Q9; 18.1’ This gives the opportunity to explore variation with time of Jan. 07 Q4, Q9(a)June 07 Bismuth half-life sheet (see file parent and daughter populations, including an example where Q5; Jan. 08 Q5 “bismuth 209 half life”) the daughter also decays. Qs 120S and file “Bismuth 209 half- Qs student book p229 life” contain some examples of calculations involving half-lives etc., and see also the relevant problem sets from Chapter 10.
18.2 The A look at the N-Z plot of nuclides: overall patterns. nuclear Nuclear stability and binding energy. valley 3-D plot of nuclides, showing how the balance of neutrons and protons relates to binding energy. Changes to nucleus in radioactive decay; decay chains. Sketch a Segre (N-Z) plot for nuclides, identifying the line of stability, and regions containing (1) alpha emitters, (2) beta minus emitters, (3) beta plus (positron) emitters, (4) fissile nuclides, (5) electron capture nuclides. Explain why the line of stability curves upwards.
03/04/2018 9:14 下午 A. M. James, Matthew Arnold School, Oxford 3 Lesson Content Activities Homework Know meaning of the terms atomic mass unit, mass defect, binding energy. Use the equation E = mc2 to convert mass to energy. Perform unit conversions kg↔u and J↔MeV. Calculate the mass defect (in u or kg) and the binding energy per nucleon (in J or MeV) for a particular nuclide, given its mass and the masses of free protons, neutrons and electrons (in kg or u). Lesson 6: Introduce N-Z (Segre) plot, noting regions Qs 170D ‘ Binding energy Qs 170D ‘ Binding energy and corresponding to stable nuclei. Discuss electrostatic and and mass defect’ mass defect’ nuclear strong forces operating in opposition, and effect of Qs 200S ‘Change in energy, Qs 200S ‘Change in energy, neutron dilution at higher values of N and Z as shown by the change in mass’ change in mass’ fact that the line of stability curves upwards. Note the regions Reading 20T ‘What holds nuclei where particular types of unstable nuclei lie in relation to the together’ line of stability. Introduce mass measurement in u, and go through sample binding energy calculation as per p231. Include definition of mass defect. Stress that binding energy per nucleon measures stability relative to free nucleons (separated sufficiently so that nuclear strong force is negligible).
Perform spreadsheet activity to plot a graph of binding energy per nucleon versus nucleon number for stable nuclides. Combine the graph obtained with the line of stability on the Segre plot to give a “3-D nuclear landscape”. Explore the 3-D nuclear landscape, identifying the various regions, including the valley bottom around iron-56. Know that all nuclear transformations involve closer approach to the deepest part of the valley floor, as this corresponds to the region with highest binding energy per nucleon. Lesson 7: Carry out spreadsheet Activity 140S to plot binding Activity 140S ‘Binding Qs 170D ‘Binding energy and energy per nucleon vs. nucleon number (A). Note that the energy of nuclei’ mass defect’ DM100S ‘Views of the Qs 200S ‘Change in energy,
03/04/2018 9:14 下午 A. M. James, Matthew Arnold School, Oxford 4 Lesson Content Activities Homework nucleons given in the file are all common, stable ones. Relate nuclear landscape’ change in mass’ the curve obtained to the Segre plot and introduce idea of a 3- Qs 170D ‘ Binding energy Predict modes of decay for D nuclear landscape with a valley of stability: the Grand and mass defect’ selected nuclei depending on Canyon is a useful analogy. Identify the various topographical Qs 200S ‘Change in energy, where they lie relative to the line of features of the nuclear landscape, as per p232: fusion hill, iron change in mass’ stability lake, Coulomb slope, Pauli cliffs, free particle plains. DM100S ‘Views of the nuclear landscape’ is useful in this respect, particularly the AVI movie clips. Discuss, in principle only at this stage, how nuclear transformations, including fission and fusion, lead to nuclei approaching the valley floor region of stability ever more closely. It may be helpful to have a binding energy per nucleon vs. A plot (upside down) to illustrate the topography of the valley floor. Identify the regions that have proton-rich and neutron-rich nuclei.
For a specific example of each of the nuclear transformations alpha emission, beta minus emission, beta plus emission, fission: Show the changes in (N, Z) on a Segre plot. Write a balanced equation for the transformation, showing how charge, baryon number, lepton number and particle number are conserved. Show, using masses and/or binding energies, that the transformation results in conversion of mass into energy. Show, using masses and/or binding energies, that the transformation leads to closer approach to the valley bottom. For beta emission, explain the changes in (N, Z) in terms of changes to neutron rich/poor nuclides. Know that gamma emission takes place when a decay process produces an excited nucleus. Know the forms of energy that are produced during decay or fission.
03/04/2018 9:14 下午 A. M. James, Matthew Arnold School, Oxford 5 Lesson Content Activities Homework Lesson 8: Details of nuclear transformations. Consider DM100S ‘Views of the Begin Qs 210S ‘Summary movement towards the valley floor in the following examples, nuclear landscape’ questions for 18.2’ illustrating by consideration of the Segre plot, which is a 2-D Extension work : Qs 190C ‘Using bird’s eye view of the landscape: (1) fission, (2) alpha decay, gamma rays’ (3) beta minus decay, (4) beta plus decay. For each type, consider a specific decay equation example, noting conservation of properties as in Chapter 17, and also note how regions of maximum stability are more closely approached by downhill transformations. Note that the apparently anomalous stability of an alpha particle, and also oxygen 16, is explained by the nuclear shell model due originally to J. H. Bartlett, which parallels the electron shell model.
Explain radioactive decay chains in terms of stepwise approach to the valley floor. Practise numerical problem solving skills involving nuclear binding energies. Lesson 9: Radioactive decay chains, considering important applications of some of the intermediate isotopes. Qs 140D is Qs 140D ‘Radioactive decay Qs 140D ‘Radioactive decay useful here. Note that each step will involve a conversion of series’ series’ mass into energy. Place in context of taking steps down into Qs 210S ‘Summary Qs 210S ‘Summary questions for the valley of stability. questions for 18.2’ 18.2’ Qs student book p235 FPP Specimen G495 Q2, Q6
18.3 Fission Discovery of nuclear fission. The fission reactor. and fusion Nuclear fusion and the possibility of a fusion reactor. Know and explain the meaning of the terms fission, sub-critical mass, critical mass, super-critical mass, chain reaction, slow neutron, moderator, absorber, enrichment. Explain how a fission chain reaction may be controlled using neutron absorbing materials to mop up excess neutrons.
03/04/2018 9:14 下午 A. M. James, Matthew Arnold School, Oxford 6 Lesson Content Activities Homework Investigate critical mass and chain reactions using software modelling activity. Explain why fission occurs in the context of movement on the binding energy valley. Calculate energy released during fission processes using data on masses and the equation E = mc2. Use data on fission process, fuel and efficiency to calculate the rate of consumption of nuclear fuel to give a specified output power. Know in brief the basic principles of operation of a fission reactor: fuel, moderator, absorber, coolant, electricity generation, safety aspects, hazards, waste containment, waste disposal and storage. Lesson 10: Discuss fission process in terms of nuclear Activity 160S ‘Nuclear Qs 250S ‘Fission and fusion : landscape and binding energies per nucleon, including a fission and critical mass’ practice questions’ specific example of a fission process. Qs 250S could be used Qs 220C ‘Life and death of a as source of data for calculations on energy released. FPP June 08 Q7; Jan. 08 nuclear reactor’ Software activity 160S can be used to illustrate fission and Q2; June 07 Q10(c) Qs 240C ‘Power in space’ critical mass. Discuss principles of operation of fission reactor, Qs 270S ‘Fission in a nuclear including details of chain reaction, moderator, absorber, reactor - how the mass changes’ coolant, safety aspects, hazards, waste containment and disposal. Note that the principles of fission reactors could be set as a homework exercise, with students pooling their findings. Know the meaning of the term fusion. 2 1/3 Use the equations E = kT, PE = e /4πε0r and r = r0A to estimate the temperature at which fusion will take place in a star. Explain why such high temperatures are needed for fusion. Explain why fusion takes place in the context of movement on the binding energy valley. Confirm that the equations involved in fusion in the Sun and experimental fusion on Earth obey the conservation laws relating to charge, baryon number,
03/04/2018 9:14 下午 A. M. James, Matthew Arnold School, Oxford 7 Lesson Content Activities Homework lepton number, particle number. Describe how fusion can be achieved in a Tokamak, explaining the roles of the electric discharge, the confining magnetic fields, the lithium blanket. Qs 250S ‘Fission and fusion: Lesson 11: Fusion. A good way to start is to set the problem practice questions’ of what temperature is needed to fuse hydrogen nuclei. This Qs 260S ‘Fusion in a kettle’ draws together aspects of Chapter 14 (ε = kT), Chapter 16 Qs 230D ‘The disappearing Sun’ 2 1/3 (PE = e /4πε0r) and Chapter 17 (r = r0A ). Note that this over- Reading 10T ‘A walk in the snow’ estimates the temperature required as being 1010 K for two Reading 20T ‘Women in nuclear protons to “kiss” and fuse, assuming ε = kT. In fact, the strong physics’ force will start to kick in well before they actually touch. Qs student book p243 Moreover, the actual temperature of the core of a star like the Sun is only 107 K. This implies ε / kT = 1000, and a vanishingly small Boltzmann factor of e-1000, too small to compute on a calculator. In fact, quantum tunneling and the action of the strong force reduce ε to about 60 times kT, with T = 107 K, implying a Boltzmann factor of e-60 = 10-26. This apparently negligible Boltzmann factor is incredibly important: it means that protons can collide billions of times a second* for thousands of years before a pair “get lucky” and fuse. Were this reaction not so slow, stars would burn up their hydrogen fuel in thousands, not billions of years, and there would be insufficient time for intelligent life to arise on the Earth to be able to discuss this very issue! Discuss fusion process in terms of conservation laws, nuclear landscape and binding energies per nucleon, including a specific example of a fusion process. Discuss fusion in Sun and in experiments such as JET project.
*Although protons are traveling very fast when the temperature is 107 K, their collision rate is little different from the gas phase collision rate of molecules at room temperature. This is because the collision cross section of a proton (10 -30 m2) is much smaller than that of a molecule (10-18 m2), so many protons whizz past each other often without interacting. Chapter 18 test Qs student book p245
03/04/2018 9:14 下午 A. M. James, Matthew Arnold School, Oxford 8 03/04/2018 9:14 下午 A. M. James, Matthew Arnold School, Oxford 9