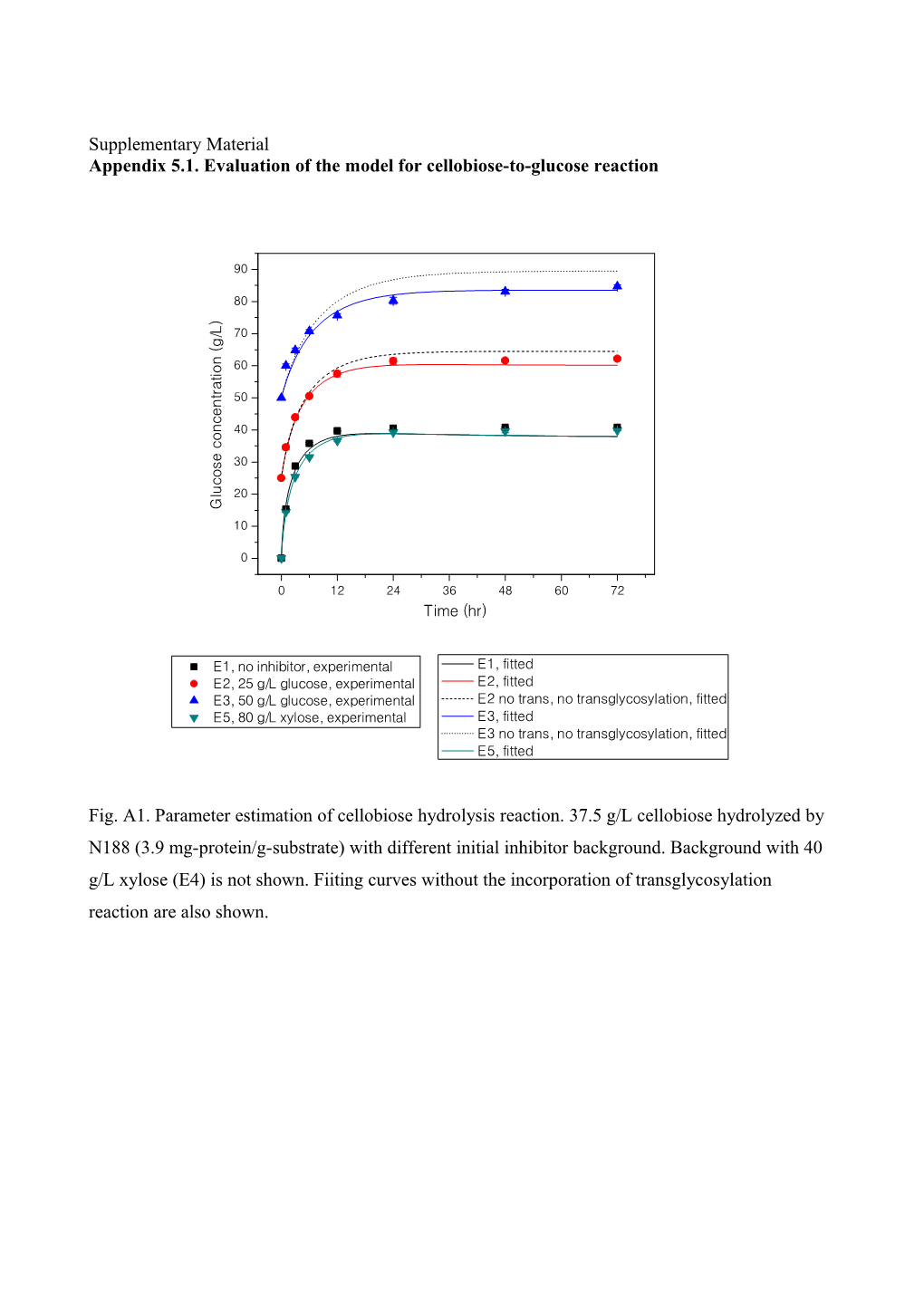
Supplementary Material Appendix 5.1. Evaluation of the model for cellobiose-to-glucose reaction
90 E1, fitted E2, fitted 80 E2 no trans, no transglycosylation, fitted ) L
/ 70 E3, fitted g (
E3 no trans, no transglycosylation, fitted n 60 o
i E5, fitted t a r
t 50 n e c
n 40 o c
e 30 s
90 o E1 mean, no inhibitor, experimental c u
l 20 90 E1, fitted E2 mean, 25 g/L glucose, experimental 80 G E2, fitted E3 mean, 50 g/L glucose, experimental 10 80 70 E2 no trans, no transglycosylation, fitted E5 mean, 80 g/L xylose, experimental
) E3, fitted L
0 / 70
60 g
( E3 no trans, no transglycosylation, fitted
n 60 E5, fitted o
50 0 i 12 24 36 48 60 72 t a r t 50 Time (hr) 40 n e c
n 40
30 o c
E1, no inhibitor,e 30 experimental E1, fitted s
20 o E2, fitted
E2, 25 g/L glucose,c experimental u E3, 50 g/L glucose,l 20 experimental E2 no trans, no transglycosylation, fitted
10 G E5, 80 g/L xylose, experimental E3, fitted 10 0 E3 no trans, no transglycosylation, fitted 0 E5, fitted 0 12 24 36 48 60 72 0 12 24 36 48 60 72 Time (hr) Fig. A1. Parameter estimation of cellobiose hydrolysis reaction. 37.5 g/L cellobiose hydrolyzed by N188 (3.9 mg-protein/g-substrate) with different initial inhibitor background. Background with 40 g/L xylose (E4) is not shown. Fiiting curves without the incorporation of transglycosylation reaction are also shown. 90
80 ) L
/ 70
g D1 (
n 60 D2 o i t D3 a r t 50 D5 n e c
n 40 o
c D1 90 e 30 90 D2 s o
80 c D3
u 80 l 20 ) D5 G L ) / 70 L g
/ 70 D1, predicted (
10 g ( n 60 D2, predicted o n i
t 60 o
i D3, predicted a 0 t r t 50 a r
n D5, predicted t 50 e n c
0 e 12 24 36 48 60 72 n
40 c o n 40 c o Time (hr) c e 30 s e
o 30 s c o u c l 20 D1, no inhibitor, experimental D1, predicted D1, no inhibitor, experimental u l G D2,20 25 g/L glucose, experimental D2, predicted
G D2, 25 g/L glucose, experimental 10 D3, 50 g/L glucose, experimental D3, predicted 10 D3, 50 g/L glucose, experimental D5, 80 g/L xylose, experimental D5, predicted 0 D5, 80 g/L xylose, experimental 0 0 12 24 36 48 60 72 0 12 24 36 48 60 72 Fig. A2. Validation of cellobioseTime (hr)hydrolysis reaction. 37.5 g/L cellobiose hydrolyzed by N188 (1.95 Time (hr) mg-protein/g-substrate) with different initial inhibitor background. Background with 40 g/L xylose (D4) is not shown. Appendix 5.2. Evaluation of Model 1 (Strategy 1)
140 130 120 110 ) L
/ 100 g (
90 n o i
t 80 a r t 70 n e
c 60 A1, predicted n o
140 c 50 A2, predicted
140 e A3, predicted 130 s 40
o 130 c 30 A4, predicted
120 u l 120
G 20 110 110 A1, no inhibitor, experimental ) )
L 10 L A2, 50 g/L glucose, experimental
/ 100 / 100 g g (
( A3, 30 g/L cellobiose, experimental
0 90
n 90 n A4, 80 g/L xylose, experimental o o i i t 80 0t 80 24 48 72 96 120 144 168 a a r r t 70 t n 70 Time (hr) n e e c 60 c 60 n n o 50 o c c
50 e e
s 40 s 40 o o c A1, predicted 30 A1,c no inhibitor, experimental u 30 u l A2,l 50 g/L glucose, experimental A2, predicted G 20 G 20 A3, 30 g/L cellobiose, experimental A3, predicted A1, no inhibitor, experimental 10 A4,10 80 g/L xylose, experimental A4, predicted A2, 50 g/L glucose, experimental 0 0 A3, 30 g/L cellobiose, experimental A4, 80 g/L xylose, experimental 0 24 48 72 0 96 24 120 48 144 72 168 96 120 144 168 Time (hr) Time (hr) Fig. A3. Validation of Model 1 (strategy 1). 100 g/L Avicel hydrolyzed by Celluclast (15.8 mg- protein/g-substrate) + N188 (5.9 mg-protein/g-substrate) with different initial inhibitor background.
90
80 B1, predicted 70 B2, predicted )
L B3, predicted /
g 60 (
B4, predicted n
o B5, predicted i
t 50
a B6, predicted r t
n B7, predicted
e 40 c n o
c 30 B1, no inhibitor, experimental e
s B2, 25 g/L glucose, experimental o
c 20
u B3, 50 g/L glucose, experimental l 90 G B4, 15 g/L cellobiose, experimental 10 B5, 30 g/L cellobiose, experimental 90 80 0 B6, 40 g/L xylose, experimental B7, 80 g/L xylose, experimental 80 0 70 24 48 72 96 120 144 168 ) L / Time (hr) 70 g 60 ( )
L n / o g i
( 60 t 50 a n r t o i t 50 n B1 a e 40 r c t B1, fitted B2 n n B1, no inhibitor, experimental o e 40
c B2, fitted c 30 B3
B2, 25 g/L glucose, experimental n e o B3, fitted B4 s B3, 50 g/L glucose, experimental c 30 o 20 c B4, fitted e B4, 15 g/L cellobiose, experimental B5 u s l o 20 B5, 30 g/L cellobiose, experimental B5, fitted B6 G c 10 u l B6, 40 g/L xylose, experimental B6, fitted B7 G 10 B7, fitted B70 , 80 g/L xylose, experimental 0 0 24 48 72 96 120 144 168 0 24 48 72 96 120 144 168 Time (hr) Fig. A4. ParameterTime estimation (hr) of Model 1 (strategy 1). 100 g/L Avicel hydrolyzed by Celluclast (10.5 mg-protein/g-substrate) with different initial inhibitor background. 100
90
80 ) L / 70 g (
n o
i 60 t a r t 50 n e c
n 40 o c
e 30 s o c u
l 20 G 10 100 100 0 90 90 0 24 48 72 96 120 144 168 ) 80
80 L / )
g Time (hr) L ( / 70
g 70 n (
o i n t o
a 60 i 60 r t t a n r t 50 e 50 n c e n c C1, predicted o C1, no inhibitor, experimental n
40 c 40 o C2, predicted c C2, 25 g/L glucose, experimental e
s e 30 C3, predicted o 30 C3, 50 g/L glucose, experimental s c o u c C4, 15 g/L cellobiose, experimental C4, predicted l u 20 l 20 G C5, 30 g/L cellobiose, experimental C5, predicted G 10 10 C6, 40 g/L xylose, experimental C6, predicted C7, 80 g/L xylose, experimental C7, predicted 0 0
0 24 480 7224 96 48 120 72 144 96 168 120 144 168 Fig. A5. ValidationTime (hr) of Model 1Time (strategy (hr) 1). 100 g/L Avicel hydrolyzed by Celluclast (21.1 mg- protein/g-substrate) with different initial inhibitor background.
90
80
70 ) L / g (
60 n o i t
a 50 r t n e
c 40 n o
c F, predicted 30 e
s G, predicted o c 20 H, predicted u l
G F, Cel / N188 = 15.8 / 3, experimental 10 G, Cel / N188 = 15.8 / 1, experimental 0 H, Cel / N188 = 31.6 / 5.9, experimental
0 24 48 72 96 120 144 168 Time (hr)
Fig. A6. Validation of Model 1 (strategy 1). 100 g/L Avicel hydrolyzed by different ratio of Celluclast/N188 loading with initial 40 g/L xylose background.
130 120 110
) 100 L / g
( 90
n o
i 80 t a r
t 70 n e
c 60 n o
c 50
e
s 40 o c
u 30 l I, 150 g/L, experimental G 20 J, 50 g/L, experimental 10 I, predicted 0 J, predicted
0 24 48 72 96 120 144 168 Time (hr) Fig. A7. Validation of Model 1 (strategy 1). 50 and 150 g/L Avicel hydrolyzed by Celluclast (15.8 mg-protein/g-substrate) + N188 (5.9 mg-protein/g-substrate). 100
90
80 ) L
/ 70 g (
n 60 o i t
a N, predicted r
t 50 n O, predicted e
c P, predicted
n 40 o
c Q, predicted
100
e 30 s
o 90 c N, no inhibitor, experimental
u 20 l
G 80 O, 50 g/L glucose, experimental 10 ) P, 30 g/L cellobiose, experimental L / 70 g Q, 80 g/L xylose, experimental (
0 n 60 o i t
0a 24 48 72 96 120 144 168 r
t 50 n
e Time (hr) c
n 40 o c 190 e 30 180 s o 170 c 20 N, no inhibitor, experimental u N, no inhibitor, experimental N, predicted 160 l G O, 50 g/L glucose, experimental O, predicted O, 50 g/L glucose, experimental 150 10 P, 30 g/L cellobiose, experimental 140 P, 30 g/L cellobiose, experimental P, predicted 130 0Q, 80 g/L xylose, experimental Q, predicted Q, 80 g/L xylose, experimental 120
) 110 0 24 48 72 96 120 144 168 L /
g 100
( Time (hr) 90 Fig.e A8. Validation of Model 1 (strategy 1). 90 g/L Barley straw hydrolyzed by Celluclast (15.8 s 80 o
c 70 u mg-protein/g-substrate)l 60 + N188 (5.9 mg-protein/g-substrate) with different initial inhibitor G background.50 N 40 O 30 20 P 10 Q 0 -10 -20 0 20 40 60 80 100 120 140 160 180 time (hr)
Appendix 5.3. Evaluation of Model 1 (Strategy 2)
130 120 110 100 ) L / 90 g (
n 80 o i t
a 70 r t n
e 60
c A1 n 50 o A2 c
e 40 A3 140 s o 130 A4 c 30
130 u l 120
G 20 120 A1, fitted 10110 110 A2, fitted ) 100 L )
/ 100 0
L A3, fitted g / (
90 g 90
( A4, fitted n 0 24 48 72 96 120 144 168 o n i t 80 80 o i a t r Time (hr) t 70 a 70 r n t e n c 60
e 60 n
c A1 o n
c 50
50 o A2 e c
s 40 e o 40 A3 s c A1, no inhibitor, experimental A1, fitted
30 o u
l A4 c 30 A2, 50 g/L glucose, experimental A2, fitted G u
20 l
G 20 A3, 30 g/L cellobiose, experimental A3, fitted 10 10 A4, 80 g/L xylose, experimental A4, fitted 0 0 0 24 48 72 96 120 144 168 Fig. A9. Parameter estimation of Model 1 (strategy 2). 100 g/L Avicel hydrolyzed by Celluclast 0 Time (hr)24 48 72 96 120 144 168 (15.8 mg-protein/g-substrate) + N188 (5.9 mg-protein/g-substrate)Time (hr) with different initial inhibitor background. 90
80
70 ) L / g (
60 n o i t
a 50
r B1 t n
e B2
c 40
n B3 o c 30 B4 e
s B5 o
c 20 B6 u l
G B7 90 10
90 80 0
80 70 0 24 48 72 96 120 144 168
70 60 Time (hr) ) ) L / L / g 60 ( 50 g
( n e o i s t 50 40 o a c r t
u B1, predicted l n B1, no inhibitor, experimental
e 40 30 G B2, predicted c B2, 25 g/L glucose, experimental n o B3, 50 g/L glucose, experimental B3, predicted c 30 20 B4, predicted e B4, 15 g/L cellobiose, experimental s o 20 10 B5, 30 g/L cellobiose, experimental B5, predicted c u B6, predicted l B6, 40 g/L xylose, experimental G 10 0 B7, 80 g/L xylose, experimental B7, predicted
0 0 24 48 72 96 120 144 168 time (hr) 0 24Fig. A10.48 Validation72 of96 Model120 1 (strategy144 2).168 100 g/L Avicel hydrolyzed by Celluclast (10.5 mg- Time (hr) protein/g-substrate) with different initial inhibitor background. 100
90
80 ) L /
g 70 (
n o i 60 t a r t
n 50 e c n
o 40 C1 c
e C2
s 30 o C3 c u l 20 C4 G 100 C5 100 10 C6 90 90 0 C7
) 80 80 L / ) 0 24 48 72 96 120 144 168 g L ( / 70 g
70 n ( Time (g/L)
o i n t o
a 60 i 60 t r t a n r t
e 50 n 50 c e n c C1, predicted o C1, no inhibitor, experimental n
40 c 40 o C2, predicted c
e C2, 25 g/L glucose, experimental
s e C3, predicted 30 o 30 C3, 50 g/L glucose, experimental s c o u
c C4, predicted
l C4, 15 g/L cellobiose, experimental u 20 20 l G C5, 30 g/L cellobiose, experimental C5, predicted G 10 10 C6, 40 g/L xylose, experimental C6, predicted C7, 80 g/L xylose, experimental C7, predicted 0 0
0 24 48 0 72 24 96 48 120 72 144 96 168 120 144 168 Fig. A11. ValidationTime of (hr) Model 1 (strategyTime (hr) 2). 100 g/L Avicel hydrolyzed by Celluclast (21.1 mg- protein/g-substrate) with different initial inhibitor background. 90 90 80 90 80 70 80 ) L / 70 g 70 (
60 n
o 60 i
t 60
a 50 r t 50 n 50 e 40 c F mean
n 40 o 40 F, predicted G mean c
30 F, predicted
e G, predicted H mean
s G, predicted 30 30 o H, predicted c 20 H, predicted u l F 20 20 G 10 F, Cel / N188 = 15.8 F, Cel / 3, / N188experimental = 15.8 / 3, experimental G 10 10 G, Cel / N188 = 15.8 G, Cel / 1, / N188experimental = 15.8 / 1, experimental H 0 H, Cel / N188 = 31.6 H, Cel / 5.9, / N188 experimental = 31.6 / 5.9, experimental 0 0 0 24 48 72 96 120 144 168 0 24 480 7224 9648 12072 14496 168120 144 168 Time (hr)
Fig. A12. Validation of Model 1 (strategy 2). 100 g/L Avicel hydrolyzed by different ratio of Celluclast/N188 loading with initial 40 g/L xylose background. 130 120 110
) 100 L / g
( 90
n o
i 80 t a r 130 t 70 140 n e
120 c 60
130 n o
c 50
120 110 e s ) 100 40
) 110 o L / c L / g u 30 I, 150 g/L, experimental l g 100 ( 90
(
G n J, 50 g/L, experimental n
90 o 20 i
o 80 i t t a a 80 r 10 r t 70 I, prediction I, predicted t n n
70 e J, predicted
e 0 J, prediction
c 60 c n n 60 o o
c 50 0 24 48 72 96 120 144 168 I c
50 e e
s J
s 40 Time (hr) o
o 40 c c
u 30 u l l 30 I G G 20 20 I, 150 g/L, experimental J 10 10 J, 50 g/L, experimental 0 0
0 024 2448 4872 7296 96120 120144 144168 168 Time (hr)Time (hr)
Fig. A13. Validation of Model 1 (strategy 2). 50 and 150 g/L Avicel hydrolyzed by Celluclast (15.8 mg-protein/g-substrate) + N188 (5.9 mg-protein/g-substrate).
100
90
80 ) L
/ 70 g (
n 60 o i t a r
t 50 n e c
n 40 o c
N
e 30
s O o
c P
u 20 l
G Q 10
0
0 24 48 72 96 120 144 168 Time (hr) 100
90
80 ) L / 70 g (
n 60 o i t a r
t 50 n e c
n 40 o c 190
e 30
180 s o 170 c 20 N, no inhibitor, experimental N, no inhibitor, experimental u N, predicted 160 l
G O, 50 g/L glucose, experimental O, predicted O, 50 g/L glucose, experimental 150 10 140 P, 30 g/L cellobiose, experimental P, predicted P, 30 g/L cellobiose, experimental 130 0Q, 80 g/L xylose, experimental Q, predicted Q, 80 g/L xylose, experimental 120
) 110
L 0 24 48 72 96 120 144 168 /
g 100 ( 90 Time (hr) e
s 80 o
Fig. A14.c 70 Validation of Model 1 (strategy 2). 90 g/L Barley straw hydrolyzed by Celluclast (15.8 u l 60 G mg-protein/g-substrate)50 + N188 (5.9 mg-protein/g-substrate) with different N initial inhibitor 40 O background.30 20 P 10 Q 0 -10 -20 0 20 40 60 80 100 120 140 160 180 time (hr)
Appendix 5.4. Evaluation of Model 2 (Gcr,tetra = 75 g/L) 130 120 110
) 100 L / g (
90 n o i
t 80 a r t 70
n A1 e
c 60 A2 n
o A3 c 140 50
e A4 s 40 130 130 o c
u 120 120 l 30 A1 G 110 20 110 A2 )
L 100 100 ) / 10 A3 L g / (
90 90 g A4 ( n
0 o n i
t 80 80 o i a t r
t 0 24 48 72 96 120 144 168 70 a 70 r n t e n c 60 Time (hr)
e 60 n
c A1 o
50 n c
50 o A2 c e
s 40
e 40 A3 o s c A1, no inhibitor, experimental A1, fitted 30 o u A4 l c 30 A2, fitted u A2, 50 g/L glucose, experimental G 20 l
G 20 A3, 30 g/L cellobiose, experimental A3, fitted 10 10 A4, 80 g/L xylose, experimental A4, fitted 0 0 0 24 48 72 96 120 144 168 0 24 48 72 96 120 144 168 Time (hr) Fig. A15. Parameter estimation of Model 2 (Gcr,tetraTime = 75 (hr) g/L). 100 g/L Avicel hydrolyzed by Celluclast (15.8 mg-protein/g-substrate) + N188 (5.9 mg-protein/g-substrate) with different initial inhibitor background. 90
80
70 ) L /
g 60 (
n o i
t 50 a r t
n B1
e 40
c B2 n o
c 30 B3
e B4 s
o 20 c B5 u 90 l B6 G 10 B7 90 80 0 80 70 0 24 48 72 96 120 144 168 70 60 Time (hr) ) ) L / L / g 50
60 g (
( n e o i s t 50 40 o a c r t
u B1, predicted l
n B1, no inhibitor, experimental
e 30 40 G B2, predicted c B2, 25 g/L glucose, experimental n o B3, 50 g/L glucose, experimental B3, predicted c 30 20 B4, predicted e B4, 15 g/L cellobiose, experimental s o 20 10 B5, 30 g/L cellobiose, experimental B5, predicted c u l B6, 40 g/L xylose, experimental B6, predicted G 10 0 B7, 80 g/L xylose, experimental B7, predicted
0 0 24 48 72 96 120 144 168 time (hr) 0 24 48 72 96 120 144 168 Fig. A16. ValidationTime (hr) of Model 2 (Gcr,tetra = 75 g/L). 100 g/L Avicel hydrolyzed by Celluclast (10.5 mg-protein/g-substrate) with different initial inhibitor background. 100
90
80 ) L /
g 70 (
n o
i 60 t C1 a r t
n 50 C2 e
c C3 n 40 o C4 c
e C5
s 30
o C6 c u l 20 C7 G
100 10 100 90 0 90
) 80 0 24 48 72 96 120 144 168
80 L / ) g
L ( Time (hr) / 70 g
70 n (
o i n t 60 o a i 60 r t t a n r t
e 50
n 50 c e n c C1, predicted o C1, no inhibitor, experimental n 40 40 c o C2, predicted
c C2, 25 g/L glucose, experimental e
s e 30 C3, predicted 30 o C3, 50 g/L glucose, experimental s c o u c C4, 15 g/L cellobiose, experimental C4, predicted l u 20 l 20 G C5, 30 g/L cellobiose, experimental C5, predicted G 10 10 C6, 40 g/L xylose, experimental C6, predicted C7, 80 g/L xylose, experimental C7, predicted 0 0
0 24 480 7224 9648 12072 144 96 168 120 144 168 Fig. A17. Validation of Model 2 (Gcr,tetra = 75 g/L). 100 g/L Avicel hydrolyzed by Celluclast (21.1 Time (hr) Time (hr) mg-protein/g-substrate) with different initial inhibitor background. 90 90 80 8090
) 70 L
/ 80 g
) 70 (
L /
n 60
g 70 o ( i
t 60 n a r o
t 50 i
t 60 n
a 50 e r t c n n 4050 e o F c
c 40
n
e G o 3040 s c F, predicted F, predicted
o 30 H e c
s G, predicted
u G, predicted
l 2030 o c G 20 H, predicted H, predicted F mean u l 20 G 10 G mean F, Cel / N188 = 15.8 / 3, experimental 10 F, Cel / N188 = 15.8 / 3, experimental H mean 100 G,G, CelCel // N188N188 == 15.815.8 // 1,1, experimentalexperimental 0 H,H, CelCel // N188N188 == 31.631.6 // 5.9,5.9, experimentalexperimental 0 0 24 48 72 96 120 144 168 0 24 48 72 96 120 144 168 0 24 48 Time72 (hr)96 120 144 168 Time (hr)
Fig. A18. Validation of Model 2 (Gcr,tetra = 75 g/L). 100 g/L Avicel hydrolyzed by different ratio of Celluclast/N188 loading with initial 40 g/L xylose background. 130 120 110
) 100 L / g (
90 n o
i 80 130 t a r 120 t 70 130n e
110 c 60 120n o )
100 c 50
L
/ 110 e g s
( 90 40
o ) 100 n c L / o u
i 80 30 I, 150 g/L, experimental l t g
( 90 a
G
r J, 50 g/L, experimental t 70 n 20 n o
i 80 e t
c 60 a 10 I, predicted r n t 70 I, prediction I o n
c 50 0 J, predicted
e J, prediction J c e 60 s 40 n o o 0 24 48 72 96 120 144 168 c
c 50
I u 30 l e Time (hr) s G 40 J
20 o c
u 30 10 l G 0 20 I, 150 g/L, experimental 10 J, 50 g/L, experimental 0 24 48 72 96 120 144 168 0 Time (hr) 0 24 48 72 96 120 144 168 Time (hr)
Fig. A19. Validation of Model 2 (Gcr,tetra = 75 g/L). 50 and 150 g/L Avicel hydrolyzed by Celluclast (15.8 mg-protein/g-substrate) + N188 (5.9 mg-protein/g-substrate). 90
80
70 ) L / g (
60 n o i t
a 50 r t n e
c 40 100 n
o N c 30 90
e O s
o P c 20 80 u
l Q ) G L / 70
10g (
N n 60 o O i
0t a
r P t 50 n Q e 0 24 48 72 96 120 144 168 c
n 40
o Time (hr) c 190
e 30
180 s o 170 c 20 N, no inhibitor, experimental N, no inhibitor, experimental u N, predicted 160 l
G O, 50 g/L glucose, experimental O, predicted O, 50 g/L glucose, experimental 150 10 140 P, 30 g/L cellobiose, experimental P, predicted P, 30 g/L cellobiose, experimental 130 0 Q, 80 g/L xylose, experimental Q, predicted Q, 80 g/L xylose, experimental 120
) 110
L 0 24 48 72 96 120 144 168 / 100 g ( 90 Time (hr)
Fig. e A20. Validation of Model 2 (Gcr,tetra = 75 g/L). 90 g/L Barley straw hydrolyzed by Celluclast
s 80 o
c 70
(15.8u mg-protein/g-substrate) + N188 (5.9 mg-protein/g-substrate) with different initial inhibitor l 60 G background.50 N 40 O 30 20 P 10 Q 0 -10 -20 0 20 40 60 80 100 120 140 160 180 time (hr) Appendix 5.5. Evaluation of Model 2 (Gcr,tetra = 80 g/L)
130 120 110
) 100 L /
g 90 (
n
o 80 i
t A1 a r
t 70 A2 n
e 60 A3 c
n A4 o 50 c
140 e s 40
o 130 A1 130 c 30 u
l A2 120 120 G 20 A3 110 110 A4
) 10
L 100 / 100 ) g L 0 ( / 90 90 g n ( o i
n 0 24 48 72 96 120 144 168 t 80 80 o a i r t t
70 a Time (hr)
n 70 r t e n c 60
e 60 n c
o A1 n
c 50
50 o A2 e c
s 40 e
o 40 A3 s c A1, no inhibitor, experimental A1, fitted
30 o u A4 l c 30 A2, fitted
u A2, 50 g/L glucose, experimental G 20 l
G 20 A3, 30 g/L cellobiose, experimental A3, fitted 10 10 A4, 80 g/L xylose, experimental A4, fitted 0 0 0 24 48 72 96 120 144 168 0 24 48 72 96 120 144 168 Time (hr) Fig. A21. Parameter estimation of Model 2 (Gcr,tetraTime = 80 (hr) g/L). 100 g/L Avicel hydrolyzed by Celluclast (15.8 mg-protein/g-substrate) + N188 (5.9 mg-protein/g-substrate) with different initial inhibitor background. 90
80
70 ) L / g (
60 n o i t
a 50
r B1 t n
e B2
c 40
n B3 o c 30 B4 e
s B5 o
c 20 B6 u l
G B7 90 10
90 80 0
80 70 0 24 48 72 96 120 144 168
70 60 Time (hr) ) ) L L / / g 50
60 g ( (
n e o s i t 50 40 o a c r t
u B1, predicted l
n B1, no inhibitor, experimental 30 e 40 G B2, predicted c B2, 25 g/L glucose, experimental n B3, predicted o B3, 50 g/L glucose, experimental c 30 20 B4, predicted e B4, 15 g/L cellobiose, experimental s o 20 10 B5, 30 g/L cellobiose, experimental B5, predicted c u B6, predicted l B6, 40 g/L xylose, experimental G 10 0 B7, 80 g/L xylose, experimental B7, predicted
0 0 24 48 72 96 120 144 168 time (hr) 0 24 48 72 96 120 144 168 Fig. A22. ValidationTime (hr)of Model 2 (Gcr,tetra = 80 g/L). 100 g/L Avicel hydrolyzed by Celluclast (10.5 mg-protein/g-substrate) with different initial inhibitor background.
100
90
80 ) L /
g C1 ( 70
n C2 o i t 60 C3 a r t
n 50 C4 e
c C5 n
o 40 C6 c
e C7 s 30 o c u l 20 C1 G C2 10 C3 100 100 C4 0 90 90 C5 0 24 48 72 96 120 144 168 C6 80 ) 80
L C7 ) / L g Time (hr) / (
70 g 70 ( n
o i n t o 60 i
60 a t r t a r n t
50 e 50 n c e n c C1, predicted o C1, no inhibitor, experimental n 40 40 c o C2, predicted e
c C2, 25 g/L glucose, experimental
s
e 30 C3, predicted 30 o C3, 50 g/L glucose, experimental s c o u C4, predicted l c C4, 15 g/L cellobiose, experimental 20 u 20 G l C5, 30 g/L cellobiose, experimental C5, predicted G C6, predicted 10 10 C6, 40 g/L xylose, experimental C7, 80 g/L xylose, experimental C7, predicted 0 0
0 24 48 0 72 24 96 48 120 72 144 96 168 120 144 168 Fig. A23. Validation of Model 2 (Gcr,tetra = 80 g/L). 100 g/L Avicel hydrolyzed by Celluclast (21.1 Time (hr) Time (hr) mg-protein/g-substrate) with different initial inhibitor background. 90 9090 80 8080
) 70 L /
g 7070 ) (
L /
n 60 g o ( i
60
t 60 n a r o t 50i F t n
a 5050 e r G t c n
n 40
e H o 40 c 40 c F, predicted
n
e 30o c s G, predicted F, predicted 30 F o 30 e c H, predicted G, predicted s G u
l 20o
c 20 H, predicted G 20 H u l F, Cel / N188 = 15.8 / 3, experimental 10G F, Cel / N188 = 15.8 / 3, experimental 1010 G, Cel / N188 = 15.8 / 1, experimental H,G, Cel Cel / / N188N188 == 31.615.8 // 5.9,1, experimental experimental 00 0 H, Cel / N188 = 31.6 / 5.9, experimental
00 2424 4848 7272 9696 120120 144144 168 0 24 48 72 96 120 144 168 Time (hr) Time (hr)
Fig. A24. Validation of Model 2 (Gcr,tetra = 80 g/L). 100 g/L Avicel hydrolyzed by different ratio of Celluclast/N188 loading with initial 40 g/L xylose background.
130 120 110 100 ) L /
g 90 (
n 80 o i t a
r 70 I, prediction t n
e 60 J, prediction c n
o 50 c
e
s 40 o c
u 30 l G 20 I, 150 g/L, experimental 10 J, 50 g/L, experimental 0
0 24 48 72 96 120 144 168 Time (hr) 130 120
130 110
) 100 L
120 / g ( 110 90 n o
i 80 t ) 100 a L / r t 70 g n ( 90
e n c 60 o n
i 80 t o a
c 50 r
t 70 e n s
e 40 o
c 60
c I n
u 30 I, 150 g/L, experimental o 50 l c J G J, 50 g/L, experimental
e 20
s 40 o
c 10 I, predicted I
u 30 l J, predicted J
G 0 20 10 0 24 48 72 96 120 144 168 0 Time (hr)
0 24 48 72 96 120 144 168 Time (hr)
Fig. A25. Validation of Model 2 (Gcr,tetra = 80 g/L). 50 and 150 g/L Avicel hydrolyzed by Celluclast (15.8 mg-protein/g-substrate) + N188 (5.9 mg-protein/g-substrate). 90
80
) 70 L / g ( 60 n o i t a
r 50 t n e
c N 40 n
o O c
e P 30 100 s
o Q c
u 90
l 20
G N 80 10 O )
L P / 70 g
0 ( Q
n 60 o i t
0 a 24 48 72 96 120 144 168 r
t 50 n
e Time (hr) c 40 n o c 190
e 30
180 s o 170 c 20 N, no inhibitor, experimental u N, no inhibitor, experimental N, predicted 160 l G O, 50 g/L glucose, experimental O, predicted O, 50 g/L glucose, experimental 150 10 P, 30 g/L cellobiose, experimental 140 P, 30 g/L cellobiose, experimental P, predicted 130 Q,0 80 g/L xylose, experimental Q, predicted Q, 80 g/L xylose, experimental 120
) 110 0 24 48 72 96 120 144 168 L / 100 g
( Time (hr) Fig. A26. 90 Parameter estimation of Model 2 (Gcr,tetra = 80 g/L). 100 g/L Avicel hydrolyzed by e
s 80 o
Celluclastc 70 (15.8 mg-protein/g-substrate) + N188 (5.9 mg-protein/g-substrate) with different initial u l 60 G inhibitor 50background. N 40 O 30 20 P 10 Q 0 -10 -20 0 20 40 60 80 100 120 140 160 180 time (hr) Appendix 5.6. Evaluation of Model 3 (Gcr,tetra = 75 g/L)
120 110 100
) 90 L / g
( 80
n o
i 70 t a r t 60 n e
c 50 A1 n
o A2 c
40
e A3 s
o 30 A4 c u l 20 140 I G 10 130 120 0 110 ) L
0 / 10024 48 72 96 120 144 168 g ( 90
n Time (hr) o i
t 80 a r t 70 n e
c 60 n o
c 50
A1, no inhibitor,e 100 g/L Avicel, experimental A1, fitted s 40 A1, no inhibitor, 100 g/L Avicel, experimental o
c A2, fitted A2, 50 g/L glucose,30 100 g/L Avicel, experimental A2, 50 g/L glucose, 100 g/L Avicel, experimental u 140 A3, 30 g/Ll cellobiose, 100 g/L Avicel, experimental A3, fitted A3, 30 g/L cellobiose, 100 g/L Avicel, experimental G 20 A1 130 A4, 80 g/L xylose, 100 g/L Avicel, experimental A4, fitted A4, 80 g/L, xylose, 100 g/L Avicel, experimental 10 A2 120 I, no inhibitor, 150 g/L Avicel, experimental I, fitted I, no inhibitor, 150 g/L Avicel, experimental 0 A3 110 A4 100 0 24 48 72 96 120 I144 168 90 Time (hr) )
L 80 Fig. / A27. Parameter estimation of Model 3 (Gcr,tetra = 75 g/L). 100 and 150 g/L Avicel hydrolyzed by g (
70 e
Celluclasts 60 (15.8 mg-protein/g-substrate) + N188 (5.9 mg-protein/g-substrate) with different initial o c
u 50 inhibitorl background. G 40 30 20 10 0
0 24 48 72 96 120 144 168 time (hr) 90
80
) 70 L / g ( 60 B1 n o
i B2 t a
r 50 B3 t n
e B4
c 40
n B5 o c
B6
e 30
s B7 o c
u 20 l B1 90 G 10 B2 B3 90 80 0 B4 B5 80 70 0 24 48 72 96 120 144 168 B6 70 60 Time (hr) B7 ) ) L / L / g
( 60 50 g
( n e o i s t 50 40 o a r c t
u B1, predicted l n B1, no inhibitor, experimental
e 40 30 G c B2, 25 g/L glucose, experimental B2, predicted n o B3, 50 g/L glucose, experimental B3, predicted c 30 20
e B4, 15 g/L cellobiose, experimental B4, predicted s o 20 10 B5, 30 g/L cellobiose, experimental B5, predicted c u l B6, 40 g/L xylose, experimental B6, predicted G 10 0 B7, 80 g/L xylose, experimental B7, predicted
0 0 24 48 72 96 120 144 168
0 24 48 72 96 120 time144 (hr) 168
Fig. A28. ValidationTime of (hr) Model 3 (Gcr,tetra = 75 g/L). 100 g/L Avicel hydrolyzed by Celluclast (10.5 mg-protein/g-substrate) with different initial inhibitor background. 100
90
) 80 L / g (
70 n o i t 60 C1 a r
t C2 n
e 50 C3 c
n C4 o
c 40 C5 e s
o 30 C6 c
u C7 l
G 20
10 100 100 0 90 90 0 24 48 72 96 120 144 168 80 ) 80 ) L / L / g Time (hr) (
g 70 70 (
n n o i t o i 60 60 a t r a t r n t
50 e n 50 c e n c C1, no inhibitor, experimental C1, predicted o n 40 40 c o C2, predicted c C2, 25 g/L glucose, experimental e
s e 30 30 C3, predicted
o C3, 50 g/L glucose, experimental s c o u c C4, 15 g/L cellobiose, experimental C4, predicted l u 20 20 l G C5, 30 g/L cellobiose, experimental C5, predicted G 10 10 C6, 40 g/L xylose, experimental C6, predicted C7, 80 g/L xylose, experimental C7, predicted 0 0
0 24 48 0 72 24 96 48 120 72 144 96 168 120 144 168 Fig. A29. ValidationTime (hr) of Model 3 (GTimecr,tetra (hr) = 75 g/L). 100 g/L Avicel hydrolyzed by Celluclast (21.1 mg-protein/g-substrate) with different initial inhibitor background. 9090
8080 90
) 70 ) 70 L / L 80 / g g (
( 60
n 60
n 70 o i o t i t a 50 r a t r 5060 t n n e e c 40 c n 4050 n o o c F, predicted
F c 30 e
s 40 G, predicted e 30 G
o F, predicted s c o 20 H, predicted H u c G, predicted l 30 u 20 l G F, Cel / N188 H, = predicted 15.8 / 3, experimental G 10 1020 G, Cel / N188 = 15.8 / 1, experimental F F, Cel / N188 = 15.8 / 3, experimental 0 H, Cel / N188 = 31.6 / 5.9, experimental G 010 G, Cel / N188 = 15.8 / 1, experimental H 0 24 H,48 Cel / N18872 = 31.696 / 5.9,120 experimental144 168 0 0 24 48 72 96 120 144 168 Time (hr) 0 24 48 Time72 (hr)96 120 144 168
Fig. A30. Validation of Model 3 (Gcr,tetra = 75 g/L). 100 g/L Avicel hydrolyzed by different ratio of Celluclast/N188 loading with initial 40 g/L xylose background.
130 120 110 130 100 120 ) L / 90
g 110
( J, 50 g/L, experimental
n 80 100 o ) i
t J, predicted L / a 70
r 90 g t ( n e 60 n 80 c o i n 50 t o a 70 r c
t J
n e 40
e 60 s c o c 30 n 50 u
o J l c
G 20
e 40 s
10 o
c 30 u 0 l
G 20 0 1024 48 72 96 120 144 168 0 Time (hr)
0 24 48 72 96 120 144 168 Time (hr) Fig. A31. Validation of Model 3 (Gcr,tetra = 75 g/L). 50 g/L Avicel hydrolyzed by Celluclast (15.8 mg-protein/g-substrate) + N188 (5.9 mg-protein/g-substrate). 90
80
) 70 L / g ( 60 n o i t a
r 50 t n e
c 40 n o c
e 30
s 100 o c
u 20 l 90 G 10 80 ) L
/ 70
0 g (
n 60 o i 0 t 24 48 72 96 120 144 168 a r
t 50 n Time (hr) e c
n 40 o c 190
e 30 180 s o 170 c 20 N, no inhibitor, experimental u N, no inhibitor, experimental N, predicted 160 l G O, 50 g/L glucose, experimental O, predicted O, 50 g/L glucose, experimental 150 10 P, 30 g/L cellobiose, experimental P, predicted P, 30 g/L cellobiose, experimental 140 Q, 80 g/L xylose, experimental 130 Q,0 80 g/L xylose, experimental Q, predicted 120
) 110 0 24 48 72 96 120 144 168 L /
g 100
( Time (hr) 90 Fig. A32.e Parameter estimation of Model 3 (Gcr,tetra = 75 g/L). 100 g/L Avicel hydrolyzed by s 80 o
c 70 u Celluclastl 60 (15.8 mg-protein/g-substrate) + N188 (5.9 mg-protein/g-substrate) with different initial G 50 N inhibitor40 background. 30 O 20 P 10 Q 0 -10 -20 0 20 40 60 80 100 120 140 160 180 time (hr) Appendix 5.7. Evaluation of Model 3 (Gcr,tetra = 80 g/L)
120
110
100 )
L 90 / g ( 80 n o i t 70 a r t
n 60 e c
n 50 o c
e 40 s o
c 30 u l 140 G 20 130 10 120 0 110 ) L
/ 100 g
0 ( 24 48 72 96 120 144 168 90 n o i
t 80 Time (hr) a r t 70 n e
c 60 n o
c 50
e A1, fitted A1, no inhibitor,s 40 100 g/L Avicel, experimental A1, no inhibitor, 100 g/L Avicel, experimental o
c A2, fitted A2, 50 g/L glucose,30 100 g/L Avicel, experimental A2, 50 g/L glucose, 100 g/L Avicel, experimental u 140 A3, 30 g/L cellobiose,l 100 g/L Avicel, experimental A3, fitted A3, 30 g/L cellobiose, 100 g/L Avicel, experimental G 20 A1 130 A4, 80 g/L xylose, 100 g/L Avicel, experimental A4, fitted A4, 80 g/L, xylose, 100 g/L Avicel, experimental 10 A2 120 I, no inhibitor, 150 g/L Avicel, experimental I, fitted I, no inhibitor, 150 g/L Avicel, experimental 0 A3 110 A4 100 0 24 48 72 96 120 I144 168 90 Time (hr)
Fig. ) A33. Parameter estimation of Model 3 (Gcr,tetra = 80 g/L). 100 and 150 g/L Avicel hydrolyzed by
L 80 / g ( Celluclast 70 (15.8 mg-protein/g-substrate) + N188 (5.9 mg-protein/g-substrate) with different initial e
s 60 o inhibitorc background.
u 50 l
G 40 30 20 10 0
0 24 48 72 96 120 144 168 time (hr) 90
80
) 70 L / g ( 60 n o i t a
r 50 B1 t
n B2 e
c 40
n B3 o c
B4
e 30
s B5 o
c B6
u 20 l
90 G B7 10 90 80 0 80 70 0 24 48 72 96 120 144 168 70 60 )
) Time (hr) L L / / g 50
60 g ( (
n e o s i t 50 40 o a c r t
u B1, predicted l
n B1, no inhibitor, experimental
e 30 40 G B2, predicted c B2, 25 g/L glucose, experimental n o B3, 50 g/L glucose, experimental B3, predicted c 30 20 B4, predicted e B4, 15 g/L cellobiose, experimental s o 20 10 B5, 30 g/L cellobiose, experimental B5, predicted c u l B6, 40 g/L xylose, experimental B6, predicted G 10 0 B7, 80 g/L xylose, experimental B7, predicted
0 0 24 48 72 96 120 144 168 Fig. A34. Validation of Model 3 (Gcr,tetra = 80 g/L). 100 g/L Avicel hydrolyzed by Celluclast (10.5 time (hr) 0 mg-protein/g-substrate)24 48 72 with96 different120 initial144 inhibitor168 background. Time (hr) 100
90
80 ) L
/ C1 g
( 70
C2 n o
i C3
t 60 a
r C4 t
n 50 C5 e c
n C6
o 40
c C7
e
s 30 o
c C1 u l 20
G C2 100 100 10 C3 C4 90 90 0 C5
80 ) 80 C6 L /
) 0 24 48 72 96 120 144 168
g C7 L / (
70
g 70 n
( Time (hr)
o i n t
o 60 a i 60 t r t a n r t 50 e 50 n c e n c C1, predicted o C1, no inhibitor, experimental n 40 40 c o C2, predicted e c C2, 25 g/L glucose, experimental
s e 30 C3, predicted 30 o C3, 50 g/L glucose, experimental s c o
u C4, predicted l c C4, 15 g/L cellobiose, experimental
u 20
20 G l C5, 30 g/L cellobiose, experimental C5, predicted G 10 10 C6, 40 g/L xylose, experimental C6, predicted C7, 80 g/L xylose, experimental C7, predicted 0 0
0 24 48 0 72 24 96 48 120 72 144 96 168 120 144 168 Time (hr) Time (hr) Fig. A35. Validation of Model 3 (Gcr,tetra = 80 g/L). 100 g/L Avicel hydrolyzed by Celluclast (21.1 mg-protein/g-substrate) with different initial inhibitor background. 9090 90 8080 80
) 70
L 70 / ) 70 g L ( / 60 g n (
60 o
i 60 n t o a
i 50 r t t
a 50 F n
r 50 t e n c 40 G e n
c 40 o 40 H c n F, predicted F, predicted
o 30 e c
s G, predicted G, predicted 3030 o e c s 20 H, predicted H, predicted u o l c
G 2020 F u
l F, Cel / N188 = 15.8 / 3, experimental 10 F, Cel / N188 = 15.8 / 3, experimental G G, Cel / N188 = 15.8 / 1, experimental G 1010 G, Cel / N188 = 15.8 / 1, experimental H, Cel / N188 = 31.6 / 5.9, experimental H 0 H, Cel / N188 = 31.6 / 5.9, experimental 00 0 24 48 72 96 120 144 168 0 24 48 72 96 120 144 168 0 24 48 Time72 (hr)96 120 144 168 Time (hr)
Fig. A36. Validation of Model 3 (Gcr,tetra = 80 g/L). 100 g/L Avicel hydrolyzed by different ratio of Celluclast/N188 loading with initial 40 g/L xylose background.
130 120 110 130 100 120 ) L / 90 110
g J, 50 g/L, experimental (
n 100
80 )
o J, predicted i L t /
a 90 70 g r ( t
n n 80
e 60 J, 50 g/L, experimental o i c t n 50 a 70 r o t
c J, predicted
n e
40 e 60 s c o n
c 30 50 o u c l
G 20 e 40 s o
10 c 30 u 0 l G 20 0 10 24 48 72 96 120 144 168 0 Time (hr) 0 24 48 72 96 120 144 168 Time (hr) Fig. A37. Validation of Model 3 (Gcr,tetra = 80 g/L). 50 g/L Avicel hydrolyzed by Celluclast (15.8 mg-protein/g-substrate) + N188 (5.9 mg-protein/g-substrate).
90
80
) 70 L / g (
n 60 o o i t
a 50 r t n e
c 40 100 n N o c 30 90 O e s
o P 80 c 20 u Q ) l L / G 70 g
10 (
n N
o 60 i 0 t O a r t 50
n P e
c 0 24 48 72 96 120 144 168 Q
n 40 o
c Time (hr) 190
e 30
180 s o 170 c N, no inhibitor, experimental N, no inhibitor, experimental u 20 N, predicted 160 l
G O, 50 g/L glucose, experimental O, predicted O, 50 g/L glucose, experimental 150 10 140 P, 30 g/L cellobiose, experimental P, predicted P, 30 g/L cellobiose, experimental Q, 80 g/L xylose, experimental 130 0 Q, predicted Q, 80 g/L xylose, experimental 120
) 110 L
/ 0 24 48 72 96 120 144 168
g 100 ( 90 Time (hr) e
s 80 o
c 70 u l 60
Fig. A38.G Parameter estimation of Model 3 (Gcr,tetra = 80 g/L). 100 g/L Avicel hydrolyzed by 50 N Celluclast40 (15.8 mg-protein/g-substrate) + N188 (5.9 mg-protein/g-substrate) O with different initial 30 P inhibitor20 background. 10 Q 0 -10 -20 0 20 40 60 80 100 120 140 160 180 time (hr) Appendix 5.8. Comparison of hydrolysis kinetics of Avicel by N188 and Xbg
90
80
) 70 L / g ( 60 n o i t
a 50 r t n e
c 40 n o c
e 30
s G, Fitted o c
u 20 l G, N188 + Cel, experimental G 10 K, Xbg + Cel, experimental
0
0 24 48 72 96 120 144 168 Time (hr)
Fig. A39. 100 g/L Avicel hydrolyzed by Celluclast (15.8 mg-protein/g-substrate) + BG (N188 or Xbg, 1 mg-protein/g-substrate) with initial 40 g/L xylose background. The dashed line is prediction of data set G by Model 2 (Gcr,tetra = 75 g/L). Appendix 5.9.Transglycosylation assay
20
) 18 L / g ( 16 n Xbg, 0.585 g/L o i t 14 Xbg, 0.293 g/L a r t
n 12 e c n
o 10 c
e 8 s o c
u 6 l g
d 4 e s
a 2 e r c
e 0 D 0 20 40 60 80 100 120 140 160 180 Glucose concentration (g/L) 20 )
L 18 / g ( 16 n
o N188, 0.585 g/L i t
a 14 N188, 0.293 g/L r t n
e 12 c n
o 10 c
e
s 8 o c u
l 6 g
d 4 e s a
e 2 r c
e 0 D 0 20 40 60 80 100 120 140 160 180 Glucose concentration (g/L)
20 ) L
/ 18 g (
n 16 o i t
a 14 r
t Cel, 1.58 g/L n
e 12 Cel, 0.585 g/L c n o
c 10
e
s 8 o c u l 6 g
d
e 4 s a e
r 2 c e
D 0
0 20 40 60 80 100 120 140 160 180 Fig. A40. Glucose concentration (g/L) Transglycosylation assay