Supplementary Material
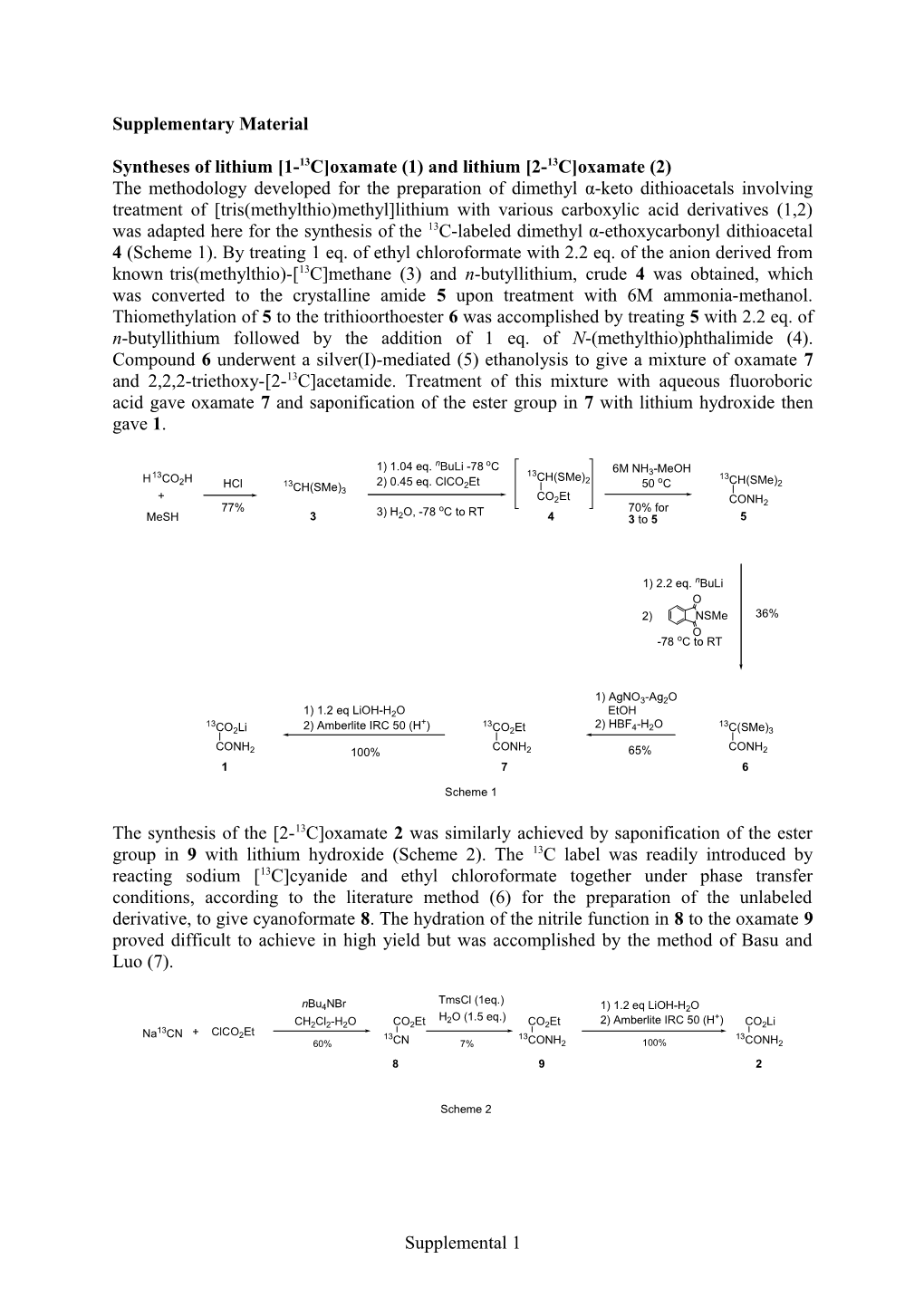
Syntheses of lithium [1-13C]oxamate (1) and lithium [2-13C]oxamate (2) The methodology developed for the preparation of dimethyl α-keto dithioacetals involving treatment of [tris(methylthio)methyl]lithium with various carboxylic acid derivatives (1,2) was adapted here for the synthesis of the 13C-labeled dimethyl α-ethoxycarbonyl dithioacetal 4 (Scheme 1). By treating 1 eq. of ethyl chloroformate with 2.2 eq. of the anion derived from known tris(methylthio)-[13C]methane (3) and n-butyllithium, crude 4 was obtained, which was converted to the crystalline amide 5 upon treatment with 6M ammonia-methanol. Thiomethylation of 5 to the trithioorthoester 6 was accomplished by treating 5 with 2.2 eq. of n-butyllithium followed by the addition of 1 eq. of N-(methylthio)phthalimide (4). Compound 6 underwent a silver(I)-mediated (5) ethanolysis to give a mixture of oxamate 7 and 2,2,2-triethoxy-[2-13C]acetamide. Treatment of this mixture with aqueous fluoroboric acid gave oxamate 7 and saponification of the ester group in 7 with lithium hydroxide then gave 1.
1) 1.04 eq. nBuLi -78 oC 6M NH -MeOH 13 13 3 H CO H CH(SMe)2 o 13CH(SMe) 2 HCl 13CH(SMe) 2) 0.45 eq. ClCO2Et 50 C 2 + 3 CO Et 2 CONH2 77% 3) H O, -78 oC to RT 70% for MeSH 3 2 4 3 to 5 5
1) 2.2 eq. nBuLi O 2) NSMe 36% O -78 oC to RT
1) AgNO3-Ag2O 1) 1.2 eq LiOH-H2O EtOH 13 + 13 2) HBF -H O 13 CO2Li 2) Amberlite IRC 50 (H ) CO2Et 4 2 C(SMe)3 CONH CONH CONH 2 100% 2 65% 2 1 7 6
Scheme 1
The synthesis of the [2-13C]oxamate 2 was similarly achieved by saponification of the ester group in 9 with lithium hydroxide (Scheme 2). The 13C label was readily introduced by reacting sodium [13C]cyanide and ethyl chloroformate together under phase transfer conditions, according to the literature method (6) for the preparation of the unlabeled derivative, to give cyanoformate 8. The hydration of the nitrile function in 8 to the oxamate 9 proved difficult to achieve in high yield but was accomplished by the method of Basu and Luo (7).
TmsCl (1eq.) nBu4NBr 1) 1.2 eq LiOH-H2O H2O (1.5 eq.) + CH2Cl2-H2O CO2Et CO2Et 2) Amberlite IRC 50 (H ) CO2Li 13 Na CN + ClCO2Et 13 13 13 60% CN 7% CONH2 100% CONH2 8 9 2
Scheme 2
Supplemental 1 Experimental All reactions involving air sensitive materials were conducted under argon. Solvents were of the highest grade commercially available. Solutions were dried over MgSO4 and evaporated at reduced pressure. Chromatography (flash) was performed on Scharlau silica gel 60 (40-60 µm). Positive ion electron impact high resolution mass spectra (+EIMS) were measured on a VG 70SE instrument at the University of Auckland, New Zealand and negative ion electrospray mass spectra (-ESMS) were recorded on a Waters Q-TOF premier tandem mass 1 spectrometer. H NMR spectra were recorded at 300 MHz in CDCl3 or DMSO d6 (internal 13 Me4Si, δ 0). C NMR spectra were recorded at 75.5 MHz in CDCl3 (center line at δ 77.0) or 13 DMSO d6 (center line at δ 39.7). C NMR assignments for the lithium salts in D2O were based on the C=O resonance values for unlabeled lithium oxamate of δ 170.5 and δ 168.9 relative to internal DSS at δ 0. DEPT experiments gave unambiguous data on the numbers of protons bonded to each carbon atom. The relative intensities (rel. int.) of the resonances in the 13C NMR are approximations.
Tris(methylthio)-[13C]methane (3) Compound 3 was prepared by the literature method3 from methanethiol (88.0 g, 1.83 mol), 13 13 H CO2H (2.0 g, 42.5 mmol, Cambridge Isotope Laboratories, Inc., 99% C) and HCl (4M in dioxane, 53 ml, 0.2 mol). The product was obtained as a colorless oil (5.1 g, 77 %). 1 1 3 H NMR (CDCl3) δ 4.66 (d, 1 H, JH-C, 162 Hz), 2.22 (d, 9 H, JH-C, 5.2 Hz). 13 1 13 C NMR (CDCl3, H- C decoupled) δ 59.2 (C, rel. int. 36), 14.8 (CH3 rel. int. 1) 13 1 13 1 C NMR (CDCl3, H- C coupled) δ 59.2 (d, JC-H 162 Hz, with fine splitting of 5 Hz), 14.8 1 (q, JC-H 140 Hz).
2,2-bis(methylthio)-[2-13C]acetamide (5) nBuLi in hexanes (1.44M, 15.2 ml, 21.9 mmol) was added to a stirred solution of compound 3 (3.24 g, 21.0 mmol) in dry THF (20 ml) cooled to -78 oC and the mixture stirred at this temperature for 1 h. A colorless precipitate formed. Ethyl chloroformate (0.92 ml, 9.54 mmol) was added over 4-5 min and after 10 min Et2O-H2O (1:1, 100 ml) was added and the mixture warmed to ambient temperature. The organic layer was separated and washed with brine, dried and the solvent evaporated to give crude ethyl 2,2-bis(methylthio)-[2-13C]acetate o (4) as an oil which was dissolved in 6M NH3-MeOH (80 ml), sealed and heated to 50 C for 16 h. The solvent was evaporated and the residue triturated with Et2O to give 5 as a colorless solid which was filtered off, washed with Et2O and dried (1.01 g, 70%). Mpt 144-145 oC. (prisms, MeOH). 1 H NMR (DMSO d6) δ 7.44 (br. s, 1 H, exchanged to D2O), 7.13 (br. d, 1 H, J 6.4 Hz, 1 3 exchanged to D2O), 4.37 (d, 1 H, JH-C 151 Hz), 2.09 (d, 6 H, JH-C 4.5 Hz). 13 1 13 1 C NMR (DMSO d6, H- C decoupled) δ 169.7 (C, d, JC-C 53 Hz, rel. int. 1), 54.6 (CH, rel. 1 int. 130), 54.6 (CH, d, JC-C 53 Hz, rel. int. 4), 13.0 (CH3, rel. int. 2). 13 1 13 1 1 C NMR (DMSO d6, H- C coupled) δ 169.7 (d, JC-C 51 Hz), 54.6 (d, rel. int. 130, JC-H 151 1 3 Hz with fine splitting of 4 Hz), 13.0 (dq, JC-H 139, JC-H 3 Hz). The multiplet expected at δ 54.6 (rel. int. 1) was not observed due to line broadening. 13 + +EIMS m/z 152.0160, CC3H9NOS2 (M) requires 152.0159.
2,2,2-tris(methylthio)-[2-13C]acetamide (6) nBuLi in hexanes (1.44M, 12.8 ml, 18.4 mmol) was added dropwise to a stirred suspension of dithioacetal 5 (1.27 g, 8.34 mmol) in dry THF (25 ml) cooled to -78 oC and the mixture stirred for 1 h. A solution of N-(methylthio)phthalimide4 (1.61 g, 8.34 mmol) in warm 1,4- dioxane (10 ml) was added in one portion (temperature rose to -40 oC). The mixture was warmed to ambient temperature and stirred for 2 h. Et2O-H2O (2:1, 100 ml) was added and
Supplemental 2 the organic layer separated, washed with brine, dried and the solvent evaporated. The residue was chromatographed on silica gel (toluene-acetone, 97:3) to give 6 (0.59 g, 36 %) as a colorless solid. o Mpt 124-125 C (plates, Et2O-hexanes). 1 H NMR (CDCl3) δ 6.94 (br.s, 1 H, exchanged to D2O), 5.66 (br.s, 1 H, exchanged to D2O), 3 2.15 (d, 9 H, JH-C 4.9 Hz). 13 1 13 1 C NMR (CDCl3 H- C decoupled) δ 170.6 (C, d, JC-C 53 Hz, rel. int. 1), 74.7 (C, rel. int. 1 186), 74.7 (C, d, JC-C 53 Hz, rel. int. 1), 13.9 (CH3, rel. int. 52). 13 1 13 1 C NMR (CDCl3 H- C coupled) δ 170.6 (d, JC-C 53 Hz), 74.7 (br. s, rel. int. 186), 13.9 (q, 1 JC-H 140 Hz). The multiplet expected at δ 74.7 (rel. int. 1) was not observed due to line broadening. 13 + +EIMS m/z 198.0034, CC4H11NOS3 (M) requires 198.0036. An alternative way to synthesize 6 would be from treatment of ethyl 2,2,2-tris(methylthio)[2- 13C]acetate with ammonia solution. In a preliminary experiment, with unlabeled material, [tris(methylthio)methyl]lithium and ethyl chloroformate were reacted together under the conditions used for synthesizing trimethyl α-keto trithioorthoesters1,2 to give ethyl 2,2,2- tris(methylthio)acetate. However this acetate failed to produce any meaningful amounts of 2,2,2-tris(methylthio)acetamide upon prolonged ammonia treatment.
Ethyl 2-amino-2-oxo-[1-13C]acetate (Ethyl [1-13C]oxamate) (7) Silver(I) oxide (1.10 g, 4.75 mmol) and silver(I) nitrate (0.403 g, 2.37 mmol)5 were added to a solution of trithioorthoester 6 (0.117 g, 0.59 mmol) in EtOH (25 ml) and the mixture stirred in the dark for 2.5 h. 1H NMR analysis of an aliquot after filtration and evaporation of the solvent revealed a mixture of desired product and mainly 2,2,2-triethoxy[2-13C]acetamide (tentatively assigned). The rest of the reaction mixture was filtered through Celite, and acidified to pH ~3 with 40% aqueous HBF4 (0.1 ml). After leaving for 2 h at ambient temperature the mixture was neutralized with Amberlyst A21 resin, filtered, and the solvent evaporated. The residue was extracted with CH2Cl2 (3 ml) and the extract applied to the top of a silica gel column and eluted with EtOAc-hexanes, 1:1 then 7:3 (with weak uv detection) to give 7 (0.045 g, 65%) as a colorless solid. 1 H NMR (CDCl3) δ 7.04 (br.s, 1 H, exchanged to D2O), 6.60 (br.s, 1 H, exchanged to D2O), 4.37 (dq, J 7.2, 3.1 Hz), 1.40 (t, 3 H, J 7.2 Hz). 13 1 13 1 C NMR (CDCl3 H- C decoupled) δ 160.3 (C, d, JC-C 82 Hz, rel. int. 1), 160.1 (C, rel. int. 1 13 25), 158.5 (C, d, JC-C 82 Hz, rel. int. 1), 63.3 (CH2, rel. int. 1), 13.9 (CH3, rel. int. 1). ( C NMR for the unlabeled compound, δ 160.1, 158.7, 63.3, 13.9). 13 1 13 3 1 C NMR (CDCl3 H- C coupled) δ 160.1 (br.d, JC-H 9 Hz), 158.5 (d, JC-C 83 Hz ), 63.3 (t, 1 1 JC-H 150 Hz), 13.9 (q, JC-H 129 Hz). The multiplet expected at δ 160.3 (rel. int. 1) was no longer observed due to line broadening. 13 + +EIMS m/z 118.0461, CC3H7NO3 (M) requires 118.0460.
Lithium 2-amino-2-oxo-[1-13C]acetate (Lithium [1-13C]oxamate) (1) Compound 7 (0.091 g, 0.77 mmol) and LiOH·H2O (0.039 g, 0.92 mmol) were stirred together in H2O (4 ml) for 30 min at ambient temperature. The pH of the mixture was adjusted to between 6-7 with Amberlite IRC 50 (H+) resin, filtered and the filtrate evaporated to leave 1 as a colorless solid (0.074 g, 100%). 13 1 13 1 C NMR (D2O, H- C decoupled) δ 170.6 (C, d, JC-C 72 Hz, rel. int. 1), 168.9 (C, rel. int. 1 30). 168.8 (C, d, JC-C 72 Hz, rel. int. 1). -ESMS m/z 89 (43%), (M-Li)-.
Ethyl [2-13C]cyanidocarbonate (Ethyl [2-13C]cyanoformate) (8)
Supplemental 3 The method described for the preparation of the unlabeled material was followed.6 Ethyl chloroformate (3.80 ml, 40.00 mmol) was added in one portion to a vigorously stirred solution of Na13CN (2.00 g, 40.00 mmol, Cambridge Isotope Laboratories, Inc., 99% 13C) and tetra-n-butylammonium bromide (0.129 g, 0.40 mmol) in a mixture of H2O (10 ml) and CH2Cl2 (10 ml) cooled in an ice-bath. The mixture was warmed to ambient temperature and stirred for 1 h. The organic layer was separated, dried and the solvent evaporated. The residue was purified by Kugelrohr distillation (130 oC/760 mmHg) to give 8 as a colorless liquid (2.39 g, 60%). The 1H and 13C NMR spectra were in agreement with the reported literature data8 for the unlabeled material except that in the 13C NMR the C=O and CN resonances 13 1 observed due to the ~1% C interactions were doublets with JC-C 112 Hz and the CN resonance due to the ~98% 13C was a singlet. The relative intensities of the 2 doublets : singlet was 1 : 70. The product had an estimated chemical purity of 90-95% and was used as such.
Ethyl 2-amino-2-oxo-[2-13C]acetate (Ethyl [2-13C]oxamate) (9) The method described below was not as successful on a gram scale. Each of seven separate flasks was charged with nitrile 8 (0.33 g, 3.29 mmol), Me3SiCl (0.42 ml, 3.29 mmol) and H2O (0.089 ml, 4.93 mmol)7 and the mixture vigorously stirred for 5 h. The contents of the flasks were all combined and the solvent evaporated. The residue was dissolved in EtOH-H2O (1:1, 5 ml) and neutralized with sat. NaHCO3 solution. Silica gel was added to absorb all the solvent then the solvent was evaporated and the residue chromatographed on silica gel (CH2Cl2-MeOH, 96:4 with weak uv detection) to give 9 as a colorless solid (0.202 g, 7%). 1 H NMR (CDCl3) δ 7.06 (br.s, 1 H), 6.67 (br.s, 1 H), 4.37 (q, 2 H, J 7.1 Hz), 1.40 (t, 3 H, J 7.1 Hz). 13 1 13 1 C NMR (CDCl3, H- C decoupled) δ 160.2 (C, d, JC-C 82 Hz, rel. int. 1), 158.7 (C, rel. int. 23), 63.3 (CH2, rel. int. 1), 13.9 (CH3). The spectrum was not sufficiently resolved to see clearly the expected doublet centered around δ 158.7 due to the ~1% 13C interactions. (13C NMR for the unlabeled compound, δ 160.1, 158.7, 63.3, 13.9).
Lithium 2-amino-2-oxo-[2-13C]acetate (Lithium [2-13C]oxamate) (2) Compound 9 (0.2 g, 1.69 mmol) and LiOH·H2O (0.085 g, 2.03 mmol) were stirred together in H2O (8 ml) for 30 min at ambient temperature. The pH of the mixture was adjusted to between 6-7 with Amberlite IRC 50 (H+) resin, filtered and the filtrate evaporated to leave 2 as a colorless solid (161 mg, 100%) contaminated with ~4% of lithium [13C]oxalate (δ 175.6) as estimated by 13C NMR. 13 1 13 1 C NMR (D2O, H- C decoupled) δ 170.6 (d, JC-C 72 Hz, rel. int. 1), 170.5 (C, rel. int. 30), 1 168.8 (d, JC-C 72 Hz, rel. int. 1).
References 1 M. Barbero, S. Cadamuro, I. Degani, S. Dughera and R. Fochi, J. Org. Chem., 1995, 60, 6017-6024. 2 I. Degani, S. Dughera, R. Fochi and E. Serra, J. Org. Chem., 1996, 61, 9572-9577. 3 R. Schlecker, U. Henkel and D. Seebach, Chem. Ber., 1977, 110, 2880-2904. 4 J. Klose, C.B. Reese and Q. Song, Tetrahedron, 1997, 53, 14411-14416. 5 A.J. Sutherland, J.K. Sutherland and P.J. Crowley, J. Chem. Soc. Perkin Trans. 1, 1996, 349-354. 6 Y. Nii, K. Okano, S. Kobayashi and M. Ohno, Tetrahedron Lett., 1979, 2517-2520. 7 M.K. Basu and F.-T. Luo, Tetrahedron Lett., 1998, 39, 3005-3006. 8 Y.A. Ibrahim, K. Kaul and N.A. Al-Awadi, Tetrahedron, 2001, 57, 10171-10176.
Supplemental 4 Acknowledgements Herbert Wong (Industrial Research Ltd.) is thanked for measuring NMR spectra and for helpful discussions.
Supplemental 5