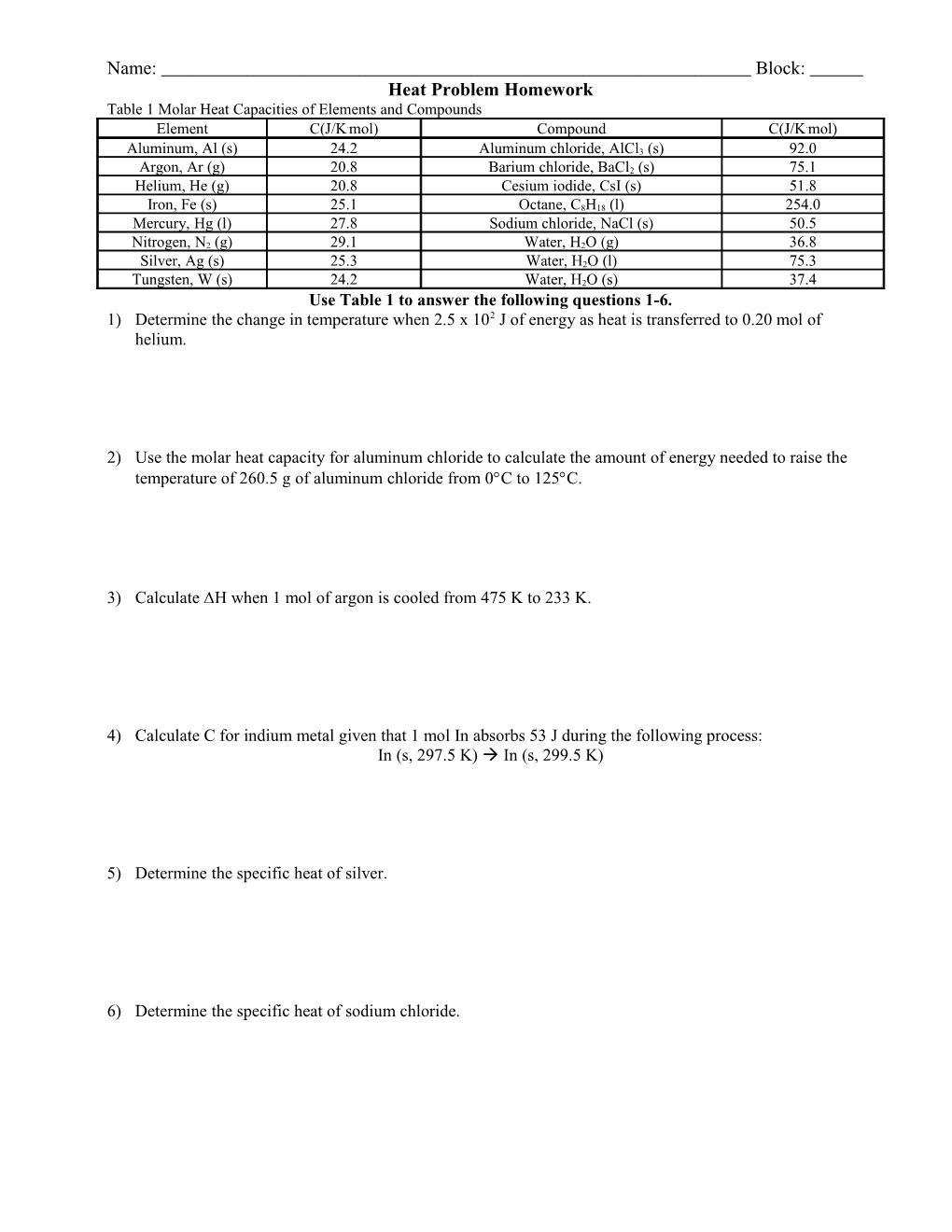
Name: Block: Heat Problem Homework Table 1 Molar Heat Capacities of Elements and Compounds Element C(J/K.mol) Compound C(J/K.mol)
Aluminum, Al (s) 24.2 Aluminum chloride, AlCl3 (s) 92.0
Argon, Ar (g) 20.8 Barium chloride, BaCl2 (s) 75.1 Helium, He (g) 20.8 Cesium iodide, CsI (s) 51.8
Iron, Fe (s) 25.1 Octane, C8H18 (l) 254.0 Mercury, Hg (l) 27.8 Sodium chloride, NaCl (s) 50.5
Nitrogen, N2 (g) 29.1 Water, H2O (g) 36.8
Silver, Ag (s) 25.3 Water, H2O (l) 75.3
Tungsten, W (s) 24.2 Water, H2O (s) 37.4 Use Table 1 to answer the following questions 1-6. 1) Determine the change in temperature when 2.5 x 102 J of energy as heat is transferred to 0.20 mol of helium.
2) Use the molar heat capacity for aluminum chloride to calculate the amount of energy needed to raise the temperature of 260.5 g of aluminum chloride from 0C to 125C.
3) Calculate H when 1 mol of argon is cooled from 475 K to 233 K.
4) Calculate C for indium metal given that 1 mol In absorbs 53 J during the following process: In (s, 297.5 K) In (s, 299.5 K)
5) Determine the specific heat of silver.
6) Determine the specific heat of sodium chloride. 7) How much heat needs to be removed to cause 112.0 g of barium chloride to experience a change of temperature from 15C to -30C if the specific heat of barium chloride is 0.36 J/K.g.
8) You need 70.2 J to raise the temperature of 34.0 g of ammonia, NH3 (g), from 23.0C to 24.0C. Calculate the specific heat of ammonia.
9) How many joules of heat are given off when 5.0 g of water cool from 75ºC to 25ºC, if the specific heat of water is 4.18 J/g.ºC?
10) How many joules does it take to melt 35 g of ice at 0ºC if the heat of fusion is 333 J/g?
11) How many calories are given off when 50 g of water at 0ºC freezes, if the heat of fusion is 79.72 cal/g?
12) a. How many calories are given off when 85 g of steam condense to liquid water? (Heat of vaporization = 539.4 cal/g)
b. Review question: How many Joules would that be?