Chapter 3 The Chemistry of Life: Organic Compounds
Lecture Outline
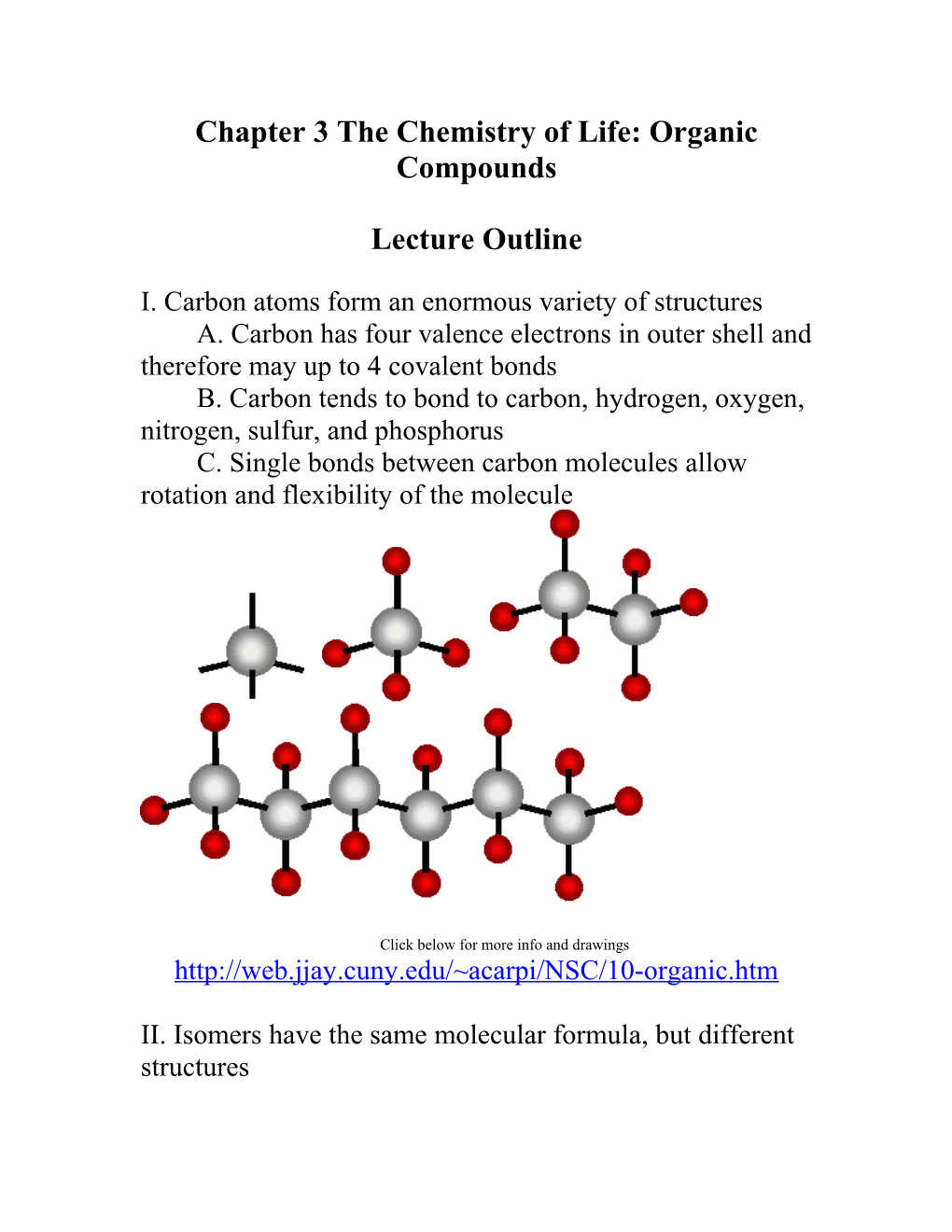
I. Carbon atoms form an enormous variety of structures A. Carbon has four valence electrons in outer shell and therefore may up to 4 covalent bonds B. Carbon tends to bond to carbon, hydrogen, oxygen, nitrogen, sulfur, and phosphorus C. Single bonds between carbon molecules allow rotation and flexibility of the molecule
Click below for more info and drawings http://web.jjay.cuny.edu/~acarpi/NSC/10-organic.htm
II. Isomers have the same molecular formula, but different structures A. Structural isomers differ in arrangement of the covalent bonds B. Geometric isomers vary in the arrangement of groups around the double bond C. Enantomers (optical) are mirror images of each other D. Single bonds allow rotation and flexibility of the molecule
III. Functional groups change the properties of organic molecules A. A hydroxyl group (R-OH) is polar
B. A carbonyl group (C=O) is polar, and characterizes aldehydes and ketones Consists of an oxygen atom joined by a double bond to a carbon atom Compo Aldehy Carboxy Acyl Acid Ketone Ester Amide Enone und de lic acid halide anhydride
Structu re
General RCOO RCONR' RC(O)C(R')C RCHO RCOR' RCOOH RCOX (RCO) O formula R' R'' R''R''' 2
Aldehyde – Carbonyl group is joined to at least one hydrogen atom Ketone – Carbonyl group is joined only to alkyl groups or aryl groups
C. A carboxyl group (R-COOH) is weakly acidic, and is an important part of amino acids
D. Amino groups (R-NH2) are weakly basic, and are an important part of amino acids
Sec Pri Ter on ma tiar dar ry y y am am am ine ine ine E. Phosphate groups (R-PO4H2) are parts of phospholipids and nucleic acids
This is the general chemical structure of an organophosphate. This is the structural formula of the phosphoric acid functional group as found in a weakly acidic aqueous solution. In more basic aqueous solutions, the group donates the two hydrogen atoms and ionizes as a phosphate group with a negative charge of 2. [4]
F. Sulfhydryl groups (R-SH) are important in some amino acids
G. Methyl groups (R-CH3) are nonpolar
IV. Many biological molecules are polymers A. Polymers are based on repeating subunits (monomers)
An amino acid monomer B. Monomers are linked by condensation reactions Keep adding amino acids and taking out water until the protein is done.
C.Polymers are degraded by hydrolysis reactions
To view a demonstration of polymer formation and degradation click the link below: http://www.phschool.com/science/biology_place/biocoach/bioprop/monomers.html
V. Carbohydrates include sugars, starches, and cellulose A. Monosaccharides are simple sugars 1. Glucose, fructose, and galactose are hexoses a. Glucose is extremely abundant and important, particularly as an energy source b. The hexoses form ring structures Monosaccharides
Three common sugars share the same molecular formula: C6H12O6. Because of their six carbon atoms, each is a hexose.
They are:
glucose, "blood sugar", the
immediate source of energy for cellular respiration galactose, a sugar in milk (and yogurt), and fructose, a sugar found in honey.
2. Deoxyribose and ribose are pentoses
Ribose Deoxyribose
B. Disaccharides consists of two monosaccharide units Maltose, lactose, and sucrose are disaccharides
Sucrose Cane sugar (sucrose) is made from glucose and fructose. C. Polysaccharides can store energy or provide structure 1. Starch is the main storage carbohydrate of plants a. Starch is a polymer of alpha-glucose b. Amylose is an unbranched starch c. Amylopectin is a branched chain, and is more common d. Plants store starch in plastids
Starches Starches are polymers of glucose. Two types are found:
amylose consists of linear, unbranched chains of several hundred glucose residues (units). The glucose residues are linked by a glycosidic bond between their #1 and #4 carbon atoms. amylopectin differs from amylose in being highly branched. At approximately every thirtieth residue along the chain, a short side chain is attached by a glycosidic bond to the #6 carbon atom (the carbon above the ring). The total number of glucose residues in a molecule of amylopectin is several thousand.
2. Glycogen is the main storage carbohydrate for animals Glycogen is primarily stored in liver and muscle cells 3. Cellulose is a structural carbohydrate Cellulose is a glucose polymer that composes cell walls 4. Most organisms cannot digest cellulose
Cellulose
Cellulose is probably the single most abundant organic molecule in the biosphere. It is the major structural material of which plants are made. Wood is largely cellulose while cotton and paper are almost pure cellulose.
D. Some modified and complex carbohydrates have special roles 1. Glucosamine makes up chitin – important in arthropods and fungal cell walls 2. Galactosamine is a component of cartilage 3. Glycoproteins and glycolipids are important molecules of the plasma membrane
VI. Lipids are fats or fatlike substances A.Fats are hydrophobic, and are composed primarily of hydrogen and oxygen B.Neutral fats contain glycerol and fatty acids
1. Neutral fats are the most abundant lipids 2. Fats are an important source of energy 3. Neutral fats are composed of a glycerol head with up to 3 fatty acid chains attached a. Unsaturated fats have one or more double bonds in the fatty acid chains 1). Unsaturated fats are typically liquid at room temperature 2). Unsaturated fats are healthier than saturated fats Not all Carbon atoms have 2 Hydrogen atoms attached C-C=C-C-C=C=C-C-H b. Saturated fats have no double bonds in the fatty acid chains 1). Saturated fats are typically solid at room temperature 2). Saturated fats are often from animal sources All Carbon atoms have 2 Hydrogen atoms C-C-C-C-C-C-H
C. Phospholipids are components of cell membranes Phospholipids are amphipathic
D. Carotenoid plant pigments are derived from isoprene units E. Steroids contain four rings of carbon atoms Steroids include cholesterol, bile salts, and hormones such as testosterone
Cholesterol F. Some chemical mediators are derived from fatty acids Prostoglandins in vertebrates and juvenile hormones of insects are examples
Figure: Classification of common phospholipids, glycolipids, and triacylglyerides VII. Proteins are macromolecules formed from amino acids A. Amino acids are the subunits of proteins 1. Amino acids contain an amino group, a carboxyl group, an alpha carbon, and a unique R group
2. There are 20 commonly occurring amino acids 3. Essential amino acids are those that must be ingested in the diet B. Peptide bonds join amino acids 1. Two amino acids form a dipeptide 2. Polypeptides are formed from more than 2 amino acids
Polypeptides are chains of amino acids. Proteins are made up of one or more polypeptide molecules.
The amino acids are linked covalently by peptide bonds.
C.Proteins have 4 levels of organization 1. Primary structure is the amino acid sequence 2. Secondary structure results from hydrogen bonding a. The alpha helix is a coiled secondary structure b. The beta-pleated sheet is formed by folding c. A single polypeptide may have portions with both types of structure 3. Tertiary structure depends on interactions among side chains R-groups interact in various ways 4. Quaternary structure results from interactions among separate polypeptide chains Hemoglobin is composed of 4 polypeptide chains
Quaternary S tructu re
D. The amino acid sequence of a protein determines its conformation Molecular chaperones may aid in the folding process in vivo E. Protein conformation is studied through a variety of methods Methods using computer technology and biotechnology allow determination of protein structure F. Protein conformation determines function 1. Regions of a protein called domains are important in the function of the protein 2. Denaturation results in disruption of the secondary, tertiary, or quaternary structure of a protein 3. Danaturation may be due to changes in pH, temperature, or various chemicals
VIII. DNA and RNA are nucleic acids A. Nucleic acids consist of nucleotide subunits Nucleotides are composed of a pentose, a phosphate group, and a nitrogenous compound
B. Some nucleotides are important in energy transfers and other cellular function 1. ATP is the energy “currency” of the cell
ATP
2. cAMP is important in cellular functioning
3. DNA and RNA are large nucleic acids important in genetics and protein synthesis