Supporting Information
Improving structural stability of MCM-41 silica nanoparticles through post-synthesis pH aging process
Mathieu Varache†, Igor Bezverkhyy†, Florence Bouyer‡, Remi Chassagnon†, Florence Baras†, Frédéric Bouyer†,*
† Laboratoire Interdisciplinaire Carnot de Bourgogne, UMR 6303 CNRS-Université de Bourgogne, 9 Avenue
Alain Savary, BP 47 870, F-21078 DIJON Cedex, France
‡ Inserm U866, Equipe Chimiothérapie, métabolisme des lipides et réponse immunitaire anti-tumorale, 7
Boulevard Jeanne d'Arc, BP 27 877, F-21078 DIJON Cedex, France
*Corresponding author : [email protected]
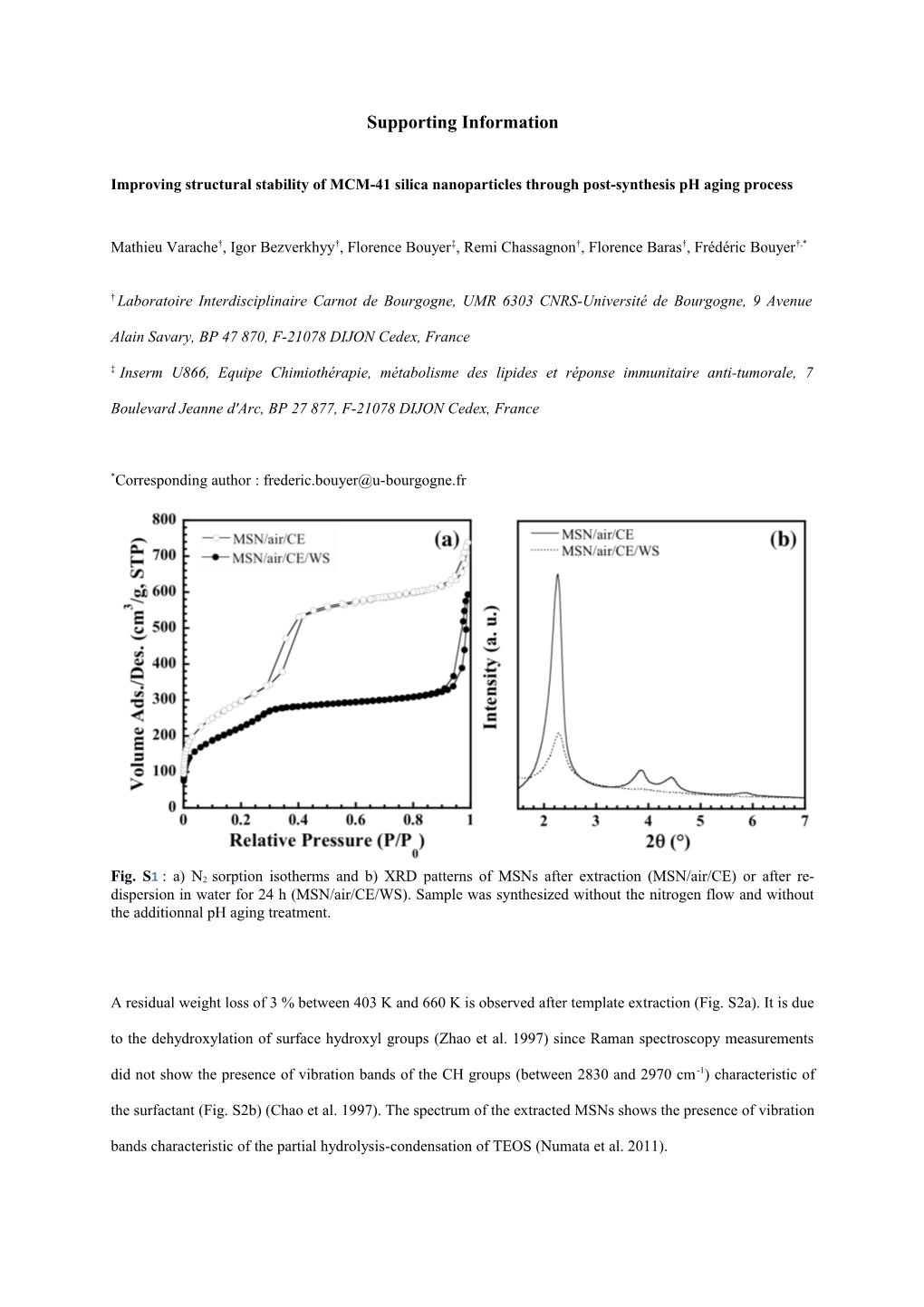
Fig. S1 : a) N2 sorption isotherms and b) XRD patterns of MSNs after extraction (MSN/air/CE) or after re- dispersion in water for 24 h (MSN/air/CE/WS). Sample was synthesized without the nitrogen flow and without the additionnal pH aging treatment.
A residual weight loss of 3 % between 403 K and 660 K is observed after template extraction (Fig. S2a). It is due to the dehydroxylation of surface hydroxyl groups (Zhao et al. 1997) since Raman spectroscopy measurements did not show the presence of vibration bands of the CH groups (between 2830 and 2970 cm -1) characteristic of the surfactant (Fig. S2b) (Chao et al. 1997). The spectrum of the extracted MSNs shows the presence of vibration bands characteristic of the partial hydrolysis-condensation of TEOS (Numata et al. 2011). Fig. S2 : Characterization of MSNs before (solid lines) and after (dotted lines) template extraction : (a) Raman spectroscopy, (b) TG analysis. The amount of CTAB in the MSNs is the difference between the weight loss of the nanoparticles before the extraction (Δm1) and after the extraction (Δm2) of the template. λex = 785 nm. The sample corresponds to a stable suspension of MSNs after synthesis and was synthesized with the additional pH aging treatment (MSN/N2/7.5).
References
Chao KJ, Wu CN, Chang H, Lee LJ, Hu S-f (1997) Incorporation of Vanadium in Mesoporous MCM-41 and Microporous AFI Zeolites. J Phys Chem B 101:6341-6349 Numata Y, Iida Y, Tanaka H (2011) Quantitative analysis of alcohol-water binary solutions using Raman spectroscopy. J Quant Spectrosc Radiat Transfer 112:1043-1049 Zhao XS, Lu GQ, Whittaker AK, Millar GJ, Zhu HY (1997) Comprehensive Study of Surface Chemistry of MCM-41 Using 29Si CP/MAS NMR, FTIR, Pyridine-TPD, and TGA. J Phys Chem B 101:6525-6531