AP Chemistry: Chapter 3 Moles and Stoichiometry September 3 - TEST
Read and interpret mass spec data and calculate atomic mass Calculate moles, grams and particles of any chemical substance Calculate percent composition Calculate empirical and molecular formulas Use dimensional analysis to solve stoichiometric problems Use dimensional analysis to solve limiting reactant problems Calculate percent yield Calculate molarity and use in solving stoichiometric problems
Atomic Masses (section 3.2)
1. Mass spectrometer – an instrument where ______or ______are passed into a beam of high-speed ______, which knock electrons off the ______or ______being analyzed and change them into ______.
Sample
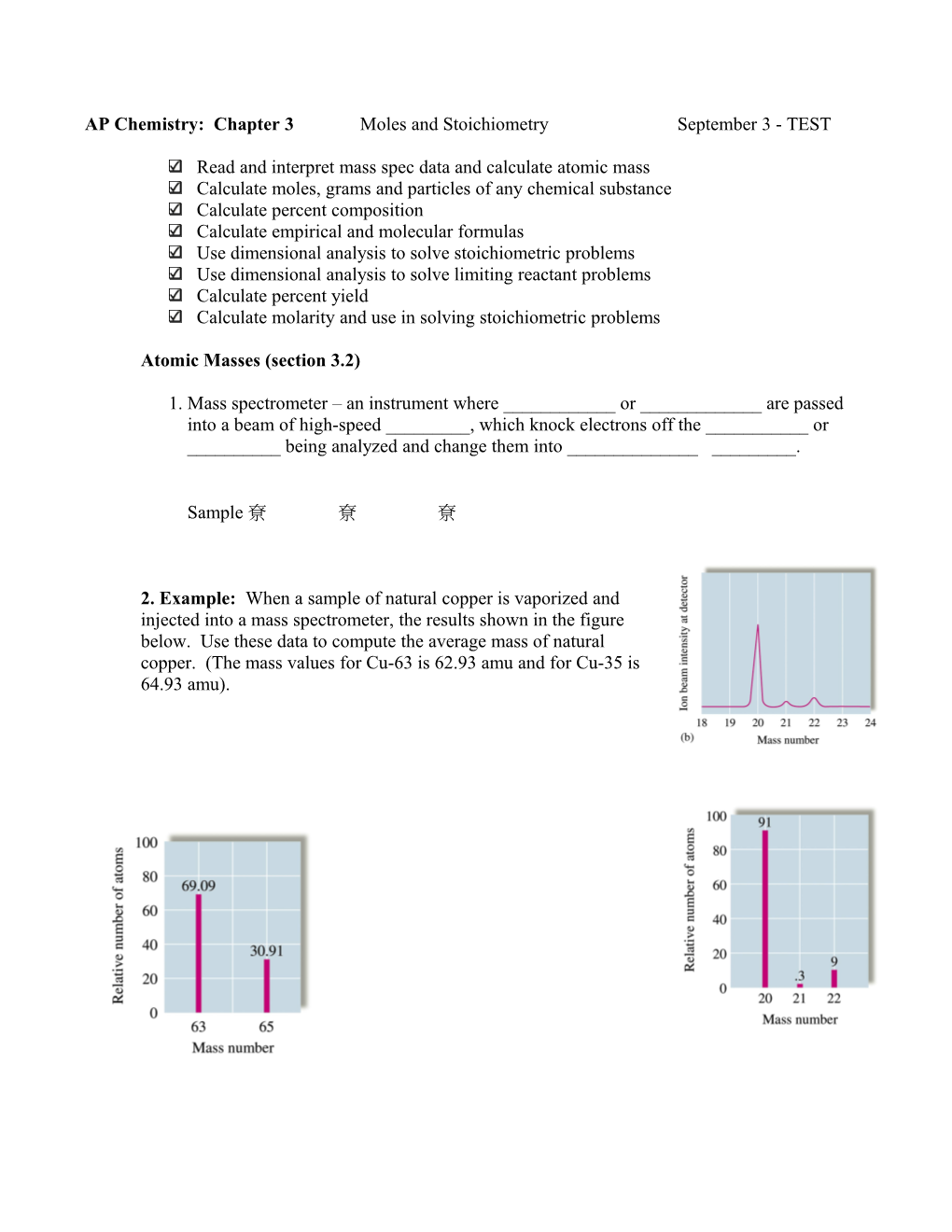
2. Example: When a sample of natural copper is vaporized and injected into a mass spectrometer, the results shown in the figure below. Use these data to compute the average mass of natural copper. (The mass values for Cu-63 is 62.93 amu and for Cu-35 is 64.93 amu). Moles (section 3.3-3.4)
1. Calculate the Molar Mass of each substance a. Cu b. Cu(NO3)2 c. CaCO3
2. How many moles of Cu are in 18.7 g of Cu?
3. How many atoms are in 127.1 g of Cu?
4. How many grams are in 2.50 moles of CaCO3?
5. How many atoms of oxygen are in 2.50 moles of Cu(NO3)2?
6. How many atoms of SF6 are in 50.0 g of SF6?
Percent Composition (section 3.5) Find the percentage composition of a chemical by finding the total molar mass of the compound find the mass percent of each element in a compound:
Example: NH4NO3
Percent Error | Observed Actual | %Error x100 Actual Empirical Formula: Rules 1. Percent to Mass 2. Mass to Moles 3. Divide by small 4. Times till whole Example: Determine the empirical formula of a compound with 52.8% Sn, 12.4% Fe, 16% C and 18.8% N. Molecular Formulas (section 3.6) 1. Molecular Formulas are ______not just the simplest ______2. the molar mass of the empirical formula (EF) is always a multiple of the MW. 3. Use the MW to determine by what factor you need to multiply the empirical formula to find the molecular formula.
Examples 1. Determine the molecular formula for a compound that contains 22.5% Na, 30.4% P and 47.1% O and a molar mass of 306 g/mol
Complex Molecular and Empirical Formula Problems—Combustion Problems 2. Many homes in rural America are heated by propane gas, a compound that contains only carbon and hydrogen. Complete combustion of a sample of propane produced 2.641 grams of carbon dioxide and 1.442 grams of water as the only products. Find the empirical formula of propane. 3. Galactose (Gal) (also called brain sugar) is a type of sugar found in dairy products, in sugar beets and other gums and mucilages. When 2.315 grams of galactose is completely burned it produces 3.953 grams of carbon dioxide and 1.389 grams of water. The molar mass of galactose is between 172 g/mol and 186 g/mol. What is the empirical and molecular formula of galactose? Stoichiometry Problems using dimensional Analysis (section 3.9) Rules 1. Always start with a balanced equation 2. Write what you know underneath the chemical that is known (5.23grams) 3. Place a question mark (?) underneath what you are looking for. 4. Use dimensional analysis to convert to the chemical you are trying to find information about. Note that you will most likely need to use the mole-mole ratio (also called the stoichiometric ratio) to convert from one chemical to another.
Examples
How many liters of hydrogen gas is formed when 13.5-grams of calcium reacts with sulfuric acid?
Nitrogen gas reacts with oxygen to form dinitrogen trioxide. How many molecules of oxygen are needed to make 5.5 L of N2O3 at STP?
Calcium Carbonate decomposes into calcium oxide and a common gas. When 45.5 grams of calcium oxide is formed how many liters of gas is also formed from this reaction. Limiting Reactant Problems (section 3.10) Rules 1. Convert all reactants to moles 2. Divide by the coefficient in the balanced equation 3. The chemical with the lowest number of moles is the limiting reactant (LR) 4. Use the LR for all further calculations
Examples:
When 114.0 g of iron and 252.7 g of chlorine gas reacts, iron(III) chloride is formed. a. Write a balanced equation b. What is the limiting reactant? c. How many grams of Iron (III) chloride is formed d. How much excess reagent is left over at the end of the experiment?
33.6 grams of sulfur dioxide reacts with 55.3 grams of water to form sulfurous acid. a. Write a balanced equation. b. What is the limiting reactant? c. How many grams of sulfurous acid will be formed? d. How much excess reagent will remain?
Percent Yield Rules 1. Balanced Equation 2. Write amount given under the reactant that has a value 3. You will be given an amount of product (write this under the product) this is the actual amount produced 4. Convert given reactant amount to same product (and units) that was given Actual Pr oduced 5. PercentYield x100 TheoreticallyMade
Examples: Nitrogen gas reacts with hydrogen gas to make ammonia (NH3). 15.5-L of N2 reacts at STP to make 30-L of ammonia. What is the percentage yield? : Three volatile compounds X, Y, and Z each contain element Q. The percent by weight of element Q in each compound was determined. Some of the data obtained are given below. Compound Percent by weight of Molecular Weight Element Q X 64.8% ? Y 73.0% 104. Z 59.3% 64.0
(a) The vapor density of compound X at 27˚C and 750. mm Hg was determined to be 3.53 grams per liter. Calculate the molecular weight of compound X. (b) Determine the mass of element Q contained in 1.00 mole of each of the three compounds. (c) Calculate the most probable value of the atomic weight of element Q. (d) Compound Z contains carbon, hydrogen, and element Q. When 1.00 gram of compound Z is oxidized and all of the carbon and hydrogen are converted to oxides, 1.37 grams of CO2 and 0.281 gram of water are produced. Determine the most probable molecular formula of compound Z.
Solution Concentrations (section 4.3) There are many ways to measure solution concentration In Chemistry the primary way is: Molarity =
Some helpful equations:
MV =
It is also a good idea to use millimoles MV= millimoles (When the volume unit is in mL)
Examples: Calculate the molarity of a solution prepared by dissolving 1.56-g of gaseous HCl into 26.8mL.
Typical blood serum is about 0.14 M NaCl. What volume of blood contains 1.00mg of NaCl?
How to Make up a solution
A 0.150 g sample of solid lead(II) nitrate is added to 125 mL of 0.100 M sodium iodide solution. Assume no change in volume of the solution. The chemical reaction that takes place is represented by the following equation. Pb(NO3)2(s) + 2 NaI(aq) → PbI2(s) + 2 NaNO3(aq)
(a) List an appropriate observation that provides evidence of a chemical reaction between the two compounds.
(b) Calculate the number of moles of each reactant.
(c) Identify the limiting reactant. Show calculations to support your identification.
(d) Calculate the molar concentration of NO3−(aq) in the mixture after the reaction is complete. (e) Circle the diagram below that best represents the results after the mixture reacts as completely as possible