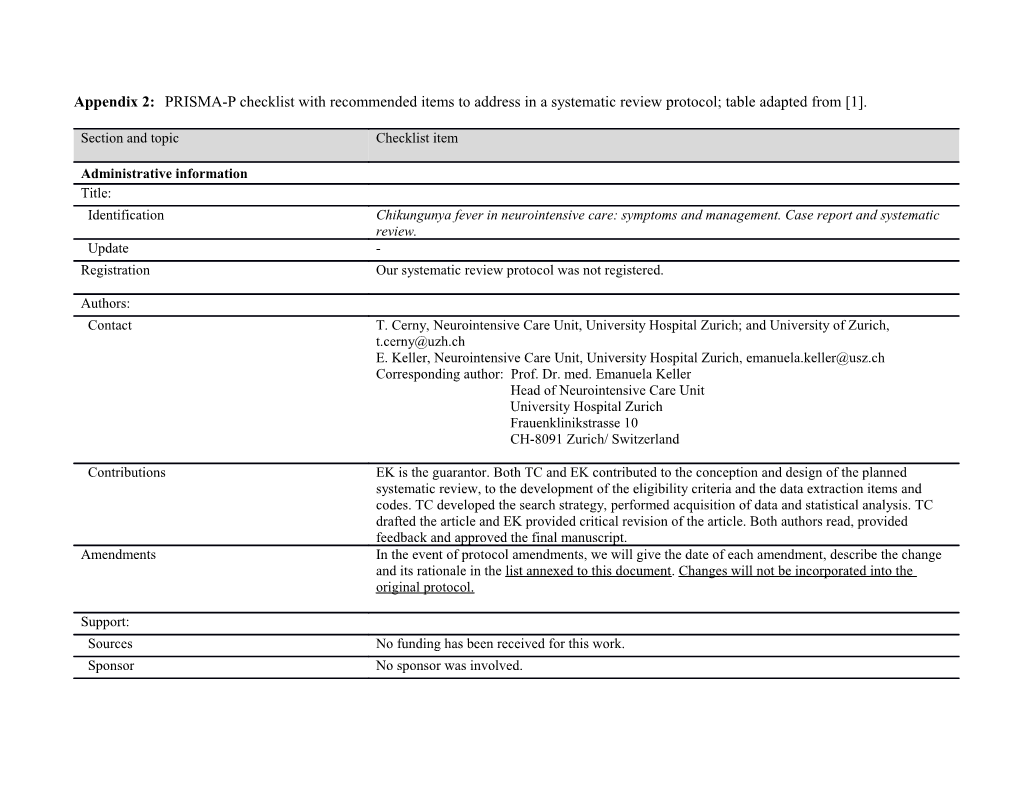
Appendix 2: PRISMA-P checklist with recommended items to address in a systematic review protocol; table adapted from [1].
Section and topic Checklist item
Administrative information Title: Identification Chikungunya fever in neurointensive care: symptoms and management. Case report and systematic review. Update - Registration Our systematic review protocol was not registered.
Authors: Contact T. Cerny, Neurointensive Care Unit, University Hospital Zurich; and University of Zurich, [email protected] E. Keller, Neurointensive Care Unit, University Hospital Zurich, [email protected] Corresponding author: Prof. Dr. med. Emanuela Keller Head of Neurointensive Care Unit University Hospital Zurich Frauenklinikstrasse 10 CH-8091 Zurich/ Switzerland
Contributions EK is the guarantor. Both TC and EK contributed to the conception and design of the planned systematic review, to the development of the eligibility criteria and the data extraction items and codes. TC developed the search strategy, performed acquisition of data and statistical analysis. TC drafted the article and EK provided critical revision of the article. Both authors read, provided feedback and approved the final manuscript. Amendments In the event of protocol amendments, we will give the date of each amendment, describe the change and its rationale in the list annexed to this document. Changes will not be incorporated into the original protocol.
Support: Sources No funding has been received for this work. Sponsor No sponsor was involved. Role of sponsor - or funder
Introduction Rationale Although Chikungunya fever (CHIKF) has been known to cause neurological symptoms since its first scientific description in the 1950s, a striking rise in the incidence of neurological manifestations has been observed following the spread of CHIKF into the Indian Ocean in 2004/05. Thereby an adapting mutation occurred, making the mosquito species Aedes albopictus a very efficient vector of Chikungunya virus (CHIKV), which in turn eased its spread in previously unaffected areas and led to a high attack rate and huge patient numbers. Now, after a decade of recurrent CHIKF outbreaks, it is evident that CHIKV is able to cause an astonishingly broad variety of neurological symptoms, some of which are extremely life-threatening in nature. Given that CHIKF will continue its spread to the Mediterranean and Gulf Coast region in the years to come, Neuro-ICUs in the western world will encounter these complications more frequently. However, to date, there is no explicit systematic review on symptoms, diagnosis and treatment of „Neuro-Chikungunya“ (CDSR and DARE retrieved Nov’15). For this reason, all existing articles reporting neurological symptoms due to CHIKF are systematically reviewed, in order to provide a sound overview of its neuropathogenic potency and possible therapeutic options. Hopefully, this will rise the awareness of Neuro-Chikungunya and foster further research on the pathophysiology and therapeutic possibilities of neurological impairment in CHIKF. Objectives To assess - the distribution and severity of central and peripheral nervous system affection in confirmed CHIKF-cases showing neurological symptoms with regard to patient age, time from disease onset and other discriminating factors becoming evident in the course of systematic case analysis. - the diagnostics deployed and therapies used in those cases - the outcome of those cases Methods Eligibility criteria PICOS (where applicable to this type of review) - Population: Patients of any age with confirmed CHIKV infection in acute care hospital setting showing neuro- logical symptoms. Confirmed CHIKV infection was defined according to laboratory criteria described by [2]. Patients with concurrent confirmed infection (such as Dengue, West Nile, Japanese encephalitis virus) or with antecedent of vaccination within one month prior to onset of symptoms were excluded. Neurological symptoms have to be reported as specific neurological status findings and/or neurological syndromes for each individual patient; ideally including information regarding patient age, time from disease onset, diagnostics and therapies deployed. Neurological symptoms excluded: - isolated paresthesia of hands (often entrapment neuropathy due to carpal tunnel syndrome caused by CHIKV-induced arthropathy). - isolated seizure in children (possibly simple febrile seizure; however, seizure Eligibility criteria included if declared as continued complex, atypical, accompanied by prolonged unconsciousness etc. and thus probably indicating CNS-infection). - Intervention: [NA] - Comparison: [NA] - Outcomes: [NA] - Study design: Case report or case series or observational study, reporting information on each individual patient regarding items such as patient age, type of neurological symptoms, time from disease onset to onset of neurological symptoms, diagnostics and therapy deployed, outcome. - Minimal requirement: Patient age AND neurological symptoms reported for every single patient.
Report characteristics: - Publication type: original article, case report, case series or scientific letter in peer reviewed journal -- note: neurological symptoms do not have to be mentioned explicitly in the article’s title or abstract, article qualifies also if dedicating a substantial part (e.g., section, denominated by subtitle) on neurological symptoms in CHIKF cases reported in the article. - Publication period: 2000 - present (~2001: emergence of relevant ECSA-strand; ~2004: adapting mutation to Ae. albopictus) - Language: English, French, German (other: if translator is found) - Publication status: only published material (e-pub, print) Information sources - Electronic databases: -- MedLine (1946 - present), Provider: PubMed and OVID -- Embase (1974 - present), Provider: EMBASE -- Cochrane library (central register) -- Web of Science -- Scopus - Other supplementary approaches: -- Scanning reference lists of articles identified as eligible -- if authors are contacted to obtain missing/unpublished data, we will state in the final article who was contacted and what kind of data was obtained. Search strategy The search strategy is derived from the eligibility criteria described above (Item 8). Limits are going to be imposed regarding publication years (2000 - present, explanation see Item 8). In contrast, no study design, publication type, language nor publication status limits will be imposed on the search, as some articles might be missed by doing so. Instead, those limits are going to be applied during the process of title/abstract and full-text screening. The following search strategy will be performed using f.ex. EMBASE: • 'chikungunya'/exp OR 'chikungunya':ab,ti AND [humans]/lim AND • 'neurologic disease'/exp OR 'neurologic disease' OR (neurol* NEAR/3 (manifest* OR complicat* OR sympt*)):ab,ti OR 'neuro chikungunya':ab,ti OR meningitis:ab,ti OR meningoencephalitis:ab,ti OR encephalitis:ab,ti OR encephalopathy:ab,ti OR cerebritis:ab,ti OR cerebellitis:ab,ti OR rhombencephalitis:ab,ti OR bickerstaff:ab,ti OR 'brain stem':ab,ti OR bulbar:ab,ti OR encephalomyelitis:ab,ti OR adem:ab,ti OR 'neuromyelitis optica':ab,ti OR myel*:ab,ti OR radicul*:ab,ti OR neuropath*:ab,ti OR 'guillain barré':ab,ti OR guillain:ab,ti OR gbs:ab,ti OR aman:ab,ti OR amsan:ab,ti OR 'polyneuritis cranialis':ab,ti OR cidp:ab,ti OR 'miller fisher':ab,ti OR mfs:ab,ti OR 'optic neuritis':ab,ti OR 'optic neuropathy':ab,ti OR 'auditory neuropathy':ab,ti OR 'vestibulocochlear neuropathy':ab,ti OR 'cranial nerve deficit':ab,ti OR 'cranial nerve involvement':ab,ti OR paralysis:ab,ti OR areflexia:ab,ti OR myoclonus:ab,ti OR ataxia:ab,ti
• Limits: publication years 2000 - present Study records: Data management All database search results will be exported to EndNote, whose “find duplicates“ function will be used to de-duplicate the records found. After completion of the selection process (see item 11b), information from the articles included in the systematic review is going to be extracted into a predefined SPSS- data file (see item 11c). IBM©-SPSS©-software (version 22) is going to be used to perform the descriptive statistical analysis and, if reasonable, search for correlations. Selection process The records yielded by the search after duplicates removed are going to be screened based on title and abstract applying the predefined eligibility criteria. Due to limited resources and given that eligibility criteria are very straightforward and broad in the case of this systematic review, the selection process will be performed by only one investigator (TC). Selected articles will be then assessed for eligibility based on full-text. The reasons for excluding articles will be stated, as will be the number of articles that could not be obtained in full-text. A summary of the whole process will be presented by means of a PRISMA flow chart. Data collection Data is going to be extracted into an a priori developed Excel- (and thereafter SPSS-) data file, which process will be pilot tested on 10 randomly selected, included studies. We will state if the extraction file was refined thereafter. Extraction will be done twice with an interval of two months by TC. In case of missing data prompting an enquiry, we will state who was how contacted and what data was obtained. Data items Following items were extracted from the articles included in the systematic review (selection of items, for a complete list of items with value codes and definitions see appendix A): - Source (author(s), publication year) - Case description (country of description/of patient origin (if travelled), case year, patient sex/age/comorbidities) - Neurological symptoms -- CNS symptoms (section affected, diagnosis, noteworthy symptoms, time from disease onset) -- PNS symptoms (section affected, diagnosis, noteworthy symptoms, time from disease onset) - Other symptoms (fever, rash, arthralgia, other atypical symptoms) - Diagnostics (other pathogens excluded, ChikV tests in blood/CSF, CSF cell count and total protein, neuropathogenic antibodies in blood/CSF, imaging, EEG, ENMG) - Therapy (interventions targeted at improving neurological symptoms, therapy response, challenges to the ICU) - Outcome (categorized as: full/partial/no recovery or lethal on the one hand, and using the modified Rankin scale on the other) Simplifications will be made in order to regroup neurological symptoms and diagnoses into a manageable and reasonable number of categories: f.ex. assumptions will be made concerning clinical jargon, such as “neck rigidity“ = meningism etc. For more details see definitions of diagnoses in Appendix A. Outcomes and prioritization There will be no outcomes in a strict sense in the planned systematic review as there will be no intervention. However, the neurological manifestations in CHIKF patients investigated in the planned review can be thought of as primary outcomes, similar to adverse effects of a drug trial. Neurological recovery and lethality can be thought of as secondary outcomes. Risk of bias in individual studies Before starting the main search, we will assess possible sources of bias in individual studies using the individual components approach [3]. We will state if the risk assessment had an influence on the subsequent data synthesis, such as exclusion of an article from the analysis, or if it prompted a subgroup analysis of the articles considered “non-biased”. Data synthesis: criteria for data Given the nature of the planned systematic review, the included studies are expected to be more or synthesis less homogenous. The required common study design - reports on patients with chikungunya fever showing neurological symptoms - is very basic and is going to be secured by the search criteria and eligibility criteria. Most data items are - if reported - comparable by nature (age, central vs. peripheral neurological manifestations, antibodies present yes/no etc.). See Item 15b and 15c for further information on management of missing values/divergent data format. summary measures Summary measures are mainly going to be the reported neurological symptoms, but encompass also & synthesis methods the other variables stated under Item 12. Data is going to be synthesized by means of descriptive statistics; examples are: - distribution pattern of neurological manifestations regarding to age group - distribution pattern of neurological manifestations regarding to time from disease onset, diagnostic markers etc. Meta-analysis is not going to be applicable to this type of systematic review. additional analyses Subgroup analyses will be performed to explore sources of heterogeneity, such as: - age, sex - time from disease onset (fever, rash, arthralgia) to occurrence of neurological symptoms - treatment etc. A narrative synthesis of the results will be provided. Meta-bias(es) An assessment of the risk of bias across studies is going to be performed before starting the main search, that is, as to how classical sources of bias (publication bias, multiple (duplicate) publication bias, location bias, citation bias, language bias, outcome reporting bias on large scale etc.) could be present in the investigation planned and what other concepts of bias have to be considered. We will state if the risk assessment had an influence on the planned data synthesis. Confidence in cumulative evidence The quality of the gathered statistical evidence is going to be discussed. The character of this discussion will be shaped by the total number of Neuro-Chikungunya cases found in the scientific literature. List of Amendments:
Date S Original protocol Revised protocol Rationale e Jan 2016 3Stating EK and TC Author added: PG (Patrick Gérardin, INSERM CIC 1410, All authors of case reports/case series a CHU de la Réunion, Saint Pierre, Reunion, France; were contacted to provide clarifications Université de La Réunion, CNRS 9192, INSERM U1187, on not stated or ambiguously stated data. IRD 249, CHU Réunion, Unité Mixte 134 Processus Infectieux en Milieu Insulaire Tropical (PIMIT), CYROI, In the case of PG and JL – both being Sainte-Clotilde, Reunion France). authors of a big case series resp. cohort PG provided extensive data, and a critical review for study – the provision of that information consistency, errors and intellectual content. PG has read, meant a workload of >10 hours. That is provided feedback and approved the final manuscript. why Co-authorship has been granted to Author added: JL (Jerôme Lemant, Intensivist, Intensive Care these two researchers. Unit, CHU de la Réunion, Saint Pierre, Reunion France). JL provided extensive data, and a critical review for consistency, errors and intellectual content. JL has read, provided feedback and approved the final manuscript. February 2016 1Subgroup analyses Case report assessment added: The systematic review of the literature 5will be performed to Before descriptive statistical analysis, all cases are assessed revealed that the quality of case reports explore sources of for quality using a clinical diagnostic algorithm. This varies quite a lot. Thus, the need for a heterogeneity algorithm classifies the case reports into three quality case report assessment became evident. categories: category A =probable Neuro-Chikungunya case, By introducing a sound assessment, the category B =plausible) and category C =disputable. Only the strength of the evidence is improved. A- and B-cases are considered for further analysis. February 2016 3Stating EK and TC Author added: US (Urs Schwarz, Neurologist, Department of US helped to develop the case report a Neurology, University Hospital Zurich, Zurich, assessment algorithm, i.e. what elements Switzerland). (clinical, diagnostical) should be US contributed to methods (case report assessment included, in which order. algorithm) and provided a critical review for consistency, errors and intellectual content. US has read, provided feedback and approved the final manuscript. February 2016 AItem did not exist New items: New items are introduced and coded p Clin pres consistent, no DD more probable (based on information from existing Clin pres consistent_described Etiol_sufficiently exclud items) so that the case report assessment algorithm (see above) can be run fully automatically within SPSS. This allows for an unbiased assessment of the case reports. March 2016 1“Chikungunya fever “The range of neurological complications in Chikungunya Adding the case report assessment (see ain neurointensive fever: case report and systematic review of 130 cases” above) puts the focus on scrutinizing the care: symptoms and existing evidence; therefore, the title is management. Case changed so that it reflects the extent of report and systematic the work. review.” July 2016 3Stating EK and TC Author added: MS (Megan Schwarz, Biologist and Virologist, Analysis of the 130 cases showed two a Department of Microbiology, Icahn School of Medicine at distinct patterns of pathomechanism Mount Sinai, New York, USA) (direct viral vs. autoimmune). We MS contributed to methods, data extraction, statistical decided to involve a virologist to analysis, results and discussion, and provided a critical perform a subgroup analysis of the review for consistency, errors and intellectual content. MS existing data set with regard to these two has read, provided feedback and approved the final categories, and to embed it within the manuscript. existing knowledge and relevant literature. August 2016 1„We will state if the Due to word count limits in the targeted publication, the Word count limits in the targeted journal 1 extraction file was statement is made here: necessitate to document this statement prefined [after pilot- We did refine the extraction data file after pilot testing it. elsewhere. testing the data file]“ Namely more categories were added in order to extract data available in the case reports to the fullest degree. Also, some categories with multiple nominal answer-classes (f.ex. “ICU- complications“: 1) ventilation 2) intubation 3) sepsis 4) arrhythmia etc.) were restructured into their subgroups so that the extracted information could be coded in a binary way (-> “ICU-ventilation“: yes/no, “ICU-intubation“: yes/no, etc.) August 2016 1“In case of missing Due to word count limits in the targeted publication, the Word count limits in the targeted journal 1 data prompting an statement is made here: necessitate to document this statement penquiry, we will state All case reports/series authors were contacted by email. We elsewhere. who was how state in the publication how many authors replied providing contacted and what additional information on how many cases. The email data was obtained.“ protocols are collected in a separate word document, which can be requested via email from the corresponding author. Appendix A: Data items, values & labels, definitions (abbreviations: n/a = not available)
Data Item Values Labels Remark/Definition
Source_pRwd 0 no 1 yes Land_descrCode 1 Austria 2 Belgium 3 China 4 Denmark 5 E 6 France mainland 7 Germany 8 Hungaria 9 Italy 10 India 11 Colombia 12 La Réunion, France overseas 13 M 14 Nepal 15 Switzerland 16 Singapore 17 Thailand 18 USA 19 UK 20 New Zealand 21 Netherlands 99 n/a missing value Case_origin 1 Gulf/Caribbean 2 South America 3 Mediterranean 4 West Africa 5 East/Central/South Africa 6 Indian Ocean 7 India/South Asia 8 Southeast Asia 9 Oceania 99 n/a missing value Case_year 99 n/a missing value Pat_sex 1 female 2 male 99 n/a missing value Pat_age 99 n/a missing value Pat_agegroup 1 newborn (<1M.) 2 infant (1-12M.) 3 child (1-12y.) 4 adolescent (13-17y.) 5 young adult (18-24y.) 6 adult (25-44y.) 7 middle aged (45-64y.) 8 aged (65+) 99 n/a missing value Pat_comorb 0 none 1 metabolic (diabetes, adipositas) 2 pulmonary (COPD, bronchiektasia) 3 cardiovascular (hypertension, smoking, past If multiple comorbidities are present including stroke or MI, then medical history pos. for MI, stroke etc.) „3=cardiovascular“ is coded. E.g. Patient with diabetes mellitus and history of myocardial infarction = cardiovascular. 4 gastrointestinal 5 hematological 6 Allergic (asthma, hay fever) 7 immunosuppressed 8 neoplasia 9 endocrinological 10 autoimmune 11 neurological (Parkinson, Alzheimer, MS) 99 n/a missing value Comorb_present 0 absent 1 present 99 no statement Adipositas 0 no 1 yes 99 n/a missing value Diabetes 0 no 1 yes 99 n/a missing value cardiovascular 0 no 1 yes 99 n/a missing value renal 0 no 1 yes 99 n/a missing value pulmonary 0 no 1 yes 99 n/a missing value allergic 0 no 1 yes 99 n/a missing value immunosuppressed 0 no 1 yes 99 n/a missing value alcoholism 0 no 1 yes 99 n/a missing value neoplasia 0 no 1 yes 99 n/a missing value neurologic 0 no 1 yes 99 n/a missing value endocrinologic 0 no 1 yes 99 n/a missing value Site_affect 1 central 2 peripheral 3 both 99 n/a missing value CNS_Dx 0 no CNS affection Define 0 as missing (Diagnosis as given by Author) 1 Meningeal syndrome MESH: A condition characterized by neck stiffness, headache, and other symptoms suggestive of meningeal irritation, but without actual [proof of] inflammation of the meninges. Core clinic: - Headache, neck stiffness - no altered mental status. Supportive clinic: - positive kernig/brudzinski sign, tense fontanelle, photophobia Diagnostics: - CSF is normal! (or: code Meningeal Syndrome if no CSF obtained) 2 Meningitis MESH: Inflammation of the coverings of the brain and/or spinal cord. Infections (viral, bacterial, and fungal) are the most common causes of this condition, but subarachnoid hemorrhage, chemical irritation, granulomatous conditions, neoplastic conditions and other inflammatory conditions may produce this syndrome. Core clinic: - same as meningeal syndrome - no altered mental status Diagnostics: - CSF is pathologic (signs of inflammation) 3 Meningoencephalitis = signs of both meningitis and encephalitis 4 Acute encephalopathy = primary or secondary brain dysfunction due to broad variety of causes: systemic infection, metabolic derangement, inherited metabolic encephalopathies, toxins, hypoxia, trauma, vasculitis, or central nervous system infection. Core clinic: - altered mental status (reduced consciousness or altered cognition, personality or behavior) code this diagnosis if no surrogate clinical markers of brain inflammation present (such as inflammatory CSF change, parenchyma inflammation on imaging). 5 Encephalitis MESH: Inflammation of the brain due to infection, autoimmune processes, toxins, and other conditions. Core clinic: (taken from the consensus statement of the International Encephalitis Consortium [4]) Major Criterion (required): - altered mental status (defined as decreased or altered level of consciousness, lethargy or personality change) lasting ≥24 h with no alternative cause identified. Minor Criteria (≥2 required1) - Documented fever ≥ 38° C (100.4°F) within the 72 h before or after presentation - Generalized or partial seizures not fully attributable to a preexisting seizure disorder2 - New onset of focal neurologic findings - CSF WBC count ≥ 5/cubic mm - Abnormality of brain parenchyma on neuroimaging suggestive of encephalitis that is either new from prior studies or appears acute in onset - Abnormality on electroencephalography that is consistent with encephalitis and not attributable to another cause
1 the IEC consensus statement subdivides encephalitis cases further according to number of minor criteria met as follows: “2 required for possible encephalitis; ≥3 required for probable or confirmed encephalitis“ [4]. For reasons of feasibility, in this review the two categories will be taken together to form the diagnosis of “encephalitis“. 2 In children, “seizure“ is considered in this review only then as a valid minor criterion if complex febrile seizures are present as a possible surrogate clinical marker for primary brain damage. Thereby, complex febrile seizures are defined as seizures with at least 1 of the following features: infant 1 year old, child 5 years old, duration more than 15 minutes, focal deficit, repeated seizures within 24 hours, focal seizures [5]. 6 Atypical encephalitis = acute febrile encephalopathy meeting the criteria of encephalitis, but with absence of focal findings on neurologic examination [6]. 7 Bickerstaff encephalitis OrphaNet ([accessed 04.12.-07.12.2015]): Bickerstaff's brainstem encephalitis (BBE) is a rare post-infectious neurological disease characterized by the association of external ophthalmoplegia, ataxia, lower limb areflexia, extensor plantar response and disturbance of consciousness (drowsiness, stupor or coma). Core Clinic: - External Ophthalmoparesis/-plegia - Ataxia - Hypersomnolence Supportive clinic: - antecedent upper respiratory or gastrointestinal tract infection - external ophthalmoplegia is progressive (within 4 weeks of onset) and relatively symmetrical - Flaccid symmetrical tetraparesis may be present (in 50%) - deep or superficial sensory impairment (lower limb areflexia) - facial weakness, bulbar palsy, internal ophthalmoplegia, blepharoptosis and nystagmus - in acute phase of disease, BBE may resemble 'brain-death' Diagnostics: - Associated with anti-GQ1b Further sources: [7]. 8 ADEM MESH and Emtree: An acute or subacute inflammatory process of the CNS characterized histologically by multiple foci of perivascular demyelination. It is believed to be a manifestation of an autoimmune attack on the myelin of the central nervous system. Symptom onset usually occurs several days after an acute viral infection or immunization, but it may coincide with the onset of infection or rarely no antecedent event can be identified. Many survivors have residual neurologic deficits. Core clinic: - subacute encephalopathy (confusion, lethargy, somnolence progressing
to coma that can be fatal) with fever and headache - multifocal neurologic symptoms (very variable, according to localization of lesions) Supportive clinic: - Interval between viral infection/immunization and symptom onset 2 days - 4 weeks Diagnostic: - MRI: multifocal lesions (hyperintens in T2, iso- or hypointens in T1) in
supra- and infratentorial white matter. If myelon affected, usually thoracal part. - CSF: very variable from normal (seldomly) to acute inflammatory: increased cell count (> 200 cells/μl possible), predominantly lymphocytic, initially neutrophil predominance possible. Total protein can be highly elevated (more than in multiple sclerosis). Further sources: [8] 9 Myelitis MESH: Inflammation of the spinal cord. Relatively common etiologies include infections, autoimmune disease, and ischemia. Core clinic: - motor weakness, sensory loss, localized pain, incontinence, other signs of autonomic dysfunction. 10 Neuromyelitis optica MESH and Emtree: A syndrome characterized by acute optic neuritis in combination with acute transverse myelitis. Demyelinating and/or necrotizing lesions form in one or both optic nerves and in the spinal cord. The onset of optic neuritis and myelitis may be simultaneous or separated by several months. - clinic: diminution of vision and possibly blindness, flaccid paralysis of the extremities, and sensory and genitourinary disturbances Further sources: [9], Dorland's Medical Dictionary, 32nd edition. 11 Myeloradiculopathy Emtree: disease of the spinal cord and spinal nerve roots; called also radiculomyelopathy. Further sources: Dorland's Medical Dictionary, 32nd edition. 12 Myeloradiculoneuropathy Disease of the spinal cord, nerve roots and nerves. 13 Encephalomyeloradiculopathy Disease of the encephalon, spinal cord and nerve roots 14 Complex febrile seizure Seizures with at least 1 of the following features: history of neurologic disease, infant <1 year old, child >5 years old, duration more than 15 minutes, neurologic deficit, repeated seizures within 24 hours, and focal seizures [5]. 99 n/a missing value CNS_section 0 no CNS affection Assignment is made based on clinical findings allowing for localization 1 Meninges and/or results of imaging, EEG, ENMG etc. 2 Encephalon 3 Cerebrum 4 Brain stem 5 Cerebellum 6 Encephalon & Myelon 7 Myelon 99 n/a CNS_Dx_corr 0a no CNS affection For Definitions see „CNS_Dx“ 1 Meningeal syndrome The CNS_Dx_corr field is used to code an alternative diagnosis if the 2 Meningitis data provided in the case report suggests another diagnosis than the one 3 Meningoencephalitis given by the author. If there is no such discrepancy evident, the same Dx 4 Acute encephalopathy is coded as in CNS_Dx. 5 Encephalitis In addition, the Dx_corr field it is used to give a diagnosis in case the 6 Atypical encephalitis original authors did not give a diagnosis. 7 Bickerstaff encephalitis 8 ADEM 9 Neuromyelitis optica 10 Myelitis 11 Myeloradiculopathy 12 Myeloradiculoneuropathy 13 Encephalomyeloradiculopathy 14 Complex febrile seizure 99 n/a missing value CNS_tfdo Time from disease onset is defined as: time from occurrence of first symptoms (fever, rash, arthralgia) to occurrence of neurological symptoms. The latter point in time can be described explicitly in the case report and can thus occur before hospitalization; if not described explicitly the day of hospitalization is used (description upon neurological examination). 99 n/a missing value PNS_Dx 0 no PNS affection Define 0 as missing (Diagnosis as given by Author) 1 Polyradiculoneuropathy, NOS MESH: Diseases characterized by injury or dysfunction involving multiple peripheral nerves and nerve roots. The process may primarily affect myelin or nerve axons. Diagnostics: CSF albuminocytological dissociation, ENMG changes 2 Classic GBS (AIDP) = Acute inflammatory demyelinating polyradiculoneuropathy = primarily demyelinating process. Core Clinic: - Weakness and areflexia/hyporeflexia in all four limbs - No/minimal ataxia - no hypersomnolence Supportive clinic: - Antecedent infectious symptoms (in up to 30% C. jejuni Gastroenteritis) - Weakness usually starts in the legs and ascends but may start in the arms [spreading to legs thereafter] - mild or moderate Weakness or complete tetraparesis - Cranial-nerves may be involved (in 50% N. facialis) - respiratory muscles may be involved - autonomous dysfunction (often subclinic) - Nadir often within 2 weeks, in 90% within 4 weeks. Diagnostics: - Electrophysiological evidence of demyelinating neuropathy (decreased nerve conduction velocity, pathologic F-waves) - CSF after 1 week: albuminocytological dissociation (with less than 10-30 cells/µl (max. 50/µl)) Sources: [10,7,11,12] 3 PCB variant of GBS Pharyngo-cervico-brachial variant Core clinic: - oropharyngeal weakness + neck weakness + arm weakness + arm areflexia/ hyporeflexia - no prominent leg weakness - No/minimal ataxia - no hypersomnolence Supportive clinic: - Antecedent infectious symptoms Diagnostics: - CSF: albuminocytological dissociation - NCS: axonal neuropathy - IgG anti-GT1a (50%) or anti-GQ1b antibodies Sources: [13,7] 4 Paraparetic GBS Core clinic: - isolated lower limbs weakness - No/minimal ataxia - no hypersomnolence Sources: [7] 5 Isolated facial GBS Official term: “Bilateral facial weakness with paraesthesias“ Core clinic: - isolated facial weakness - No/minimal ataxia - no hypersomnolence Sources: [7] 6 AMAN Core clinic: - like classic GBS, but faster and more severe - only motor deficits Diagnostics: - ENMG: primarily axonal neuropathy - GM1-Ak Sources: [14,12] 7 AMSAN Core clinic: - like classic GBS, but faster and more severe - sensory and motor deficits Diagnostics: - ENMG: primarily axonal neuropathy - GM1-Ak Sources: [14,12] 8 Polyneuritis cranialis Core clinic: - oropharyngeal weakness (~PCB) with ophthalmoparesis (MFS) - No ataxia (unlike MFS!) - No hypersomnolence Supportive clinic: - bilateral facial palsy Sources: [15,16] 9 Miller-Fisher-Syndrome MESH: A variant of the GBS-spectrum characterized by acute onset of oculomotor dysfunction, ataxia, and loss of deep tendon reflexes. Core clinic: - Ophthalmoparesis/-plegia and a-/hyporeflexia - ataxia (due to peripheral sensory nerve dysfunction) - No hypersomnolence - Absence of limb/trunk weakness (Presence of limb weakness = MFS-GBS-Overlap) Supportive clinic: - Absence of certain features indicates incomplete MFS - Facial weakness and sensory loss may also occur - ataxia is produced by peripheral sensory nerve dysfunction and not by cerebellar injury Diagnostics: - ENMG: axonal neuropathy - GQ1b-Ak in 95% present Further sources: [14,7] 10 MFS-GBS-Overlap Core clinic: - Ophthalmoparesis/-plegia and a-/hyporeflexia - ataxia (peripheral sensory nerve dysfunction) - No hypersomnolence - Presence of limb weakness 11 CIDP = chronic inflammatory demyelinating polyradiculoneuropathy Core clinic: slowly progressive paresis / paresthesia of proximal legs Sources: [14,12] 12 MMN = Multifocal motor neuropathy: chronic autoimmune-inflammatory, purely motoric neuropathy Core clinic: - weakness, muscle atrophy and cramping mostly of the arms, usually beginning in one or both hands - asymmetric Diagnostics: - ENMG shows conduction blocks of motor nerves Sources: [14,12] 13 MADSAM = multifocal acquired demyelinating sensory and motor neuropathy (Lewis-Sumner-Syndrom): chronic autoimmune-inflammatory, sensory and motor neuropathy Core clinic: - asymmetric paresis and hypesthesias Sources: [14,12] 14 Optic neuritis MESH: Inflammation of the optic nerve. Commonly associated conditions include autoimmune disorders, infections, and granulomatous diseases. Inflammation may occur in the portion of the nerve within the globe (neuropapillitis or anterior optic neuritis) or the portion behind the globe (retrobulbar neuritis or posterior optic neuritis). Core clinic: - defective vision (color vision, contrast sensitivity) that may progress to severe visual loss - retro-orbital pain that is aggravated by eye movement Diagnostics: - RAPD, Fundus (optic disc hyperemi, edema etc.), Visual field defects, Visual evoked potentials etc. 15 Neuroretinitis Def: A vascular opticus process: vasculitis of prelaminary arterioles of the papilla with secondarily spreading submacular transudate and stellate lipid deposits. Idiopathic neuroretinitis is caused by a broad variety of infectious and non-infectious intraocular as well as systemic diseases. Its pathogenetic common ground is a local or generalized (post-)infectious immunvasculitis, mediated by pathogen-specific mechanisms. [Bialasiewicz, AA. (2000), Neuroretinitis, Ophthalmologe 2000; 97: 374–391.] Core clinic: - uni- or bilaterally defective vision (acuity, color vision, contrast sensitivity) - in 50% Fever, fatigue, night sweat - possibly retro-orbital pain, aggravated by eye movement Diagnostics: - RAPD, Fundus, Visual field defects, Fluorescein Angiography, Electroretinogram, VEPs etc. Sources: [17] 16 Auditory neuropathy Emtree: auditory neuropathy = neuropathy in the eighth cranial nerve with apparently normal function of the outer hair cells but dysfunction of the inner hair cells or cochlear nerve. 17 Peripheral polyneuropathy Emtree: neuropathy of several peripheral nerves simultaneously; called also multiple or peripheral neuropathy. 18 Neuromyelitis optica MESH and Emtree: A syndrome characterized by acute optic neuritis in combination with acute transverse myelitis. Demyelinating and/or necrotizing lesions form in one or both optic nerves and in the spinal cord. The onset of optic neuritis and myelitis may be simultaneous or separated by several months. Core clinic: - diminution of vision and possibly blindness - flaccid paralysis of the extremities - sensory and genitourinary disturbances Further sources: [9], Dorland's Medical Dictionary, 32nd edition. 19 Myeloradiculopathy Emtree: disease of the spinal cord and spinal nerve roots; called also radiculomyelopathy. 20 Myeloradiculoneuropathy = disease of the spinal cord, nerve roots and nerves. 21 Hypokalemic paralysis = acute-onset flaccid paralysis associated with low plasma potassium (<3.5 mEq/L). Hypokalemic paralysis can be idiopathic (e.g. familial hypokalemic paralysis) or secondary. Secondary hypokalemic paralysis is usually associated with renal (distal RTA) and gastrointestinal disorders, furthermore with thyrotoxicosis, dengue viral infection, Gitelman syndrome, and Conn's syndrome. Source: [18] 22 Encephalomyeloradiculopathy disease of the encephalon, spinal cord and nerve roots 99 n/a missing value PNS_section 0 no PNS affection Assignment is made based on clinical findings allowing for localization 1 Radiculopathy and/or results of imaging, EEG, ENMG etc. 2 Radiculoneuropathy 3 Radiculoneuropathy incl. cranial nerves 4 Neuropathy of cranial nerves 5 Peripheral neuropathy 6 Myopathy 99 n/a missing value PNS_Dx_corr 0 no PNS affection For Definitions see „PNS_Dx“. 1 GBS-Spectrum, NOS The PNS_Dx_corr field is used to code an alternative diagnosis if the 2 Classic GBS (AIDP) data provided in the case report suggests another diagnosis than the one 3 PCB variant of GBS given by the author. If there is no such discrepancy evident, the same Dx 4 Paraparetic GBS is coded as in PNS_Dx. 5 Isolated facial GBS In addition, the Dx_corr field it is used to give a diagnosis in case the 6 AMAN original authors did not give a diagnosis. 7 AMSAN 8 Polyneuritis cranialis 9 Miller-Fisher-Syndrome 10 MFS-GBS-Overlap 11 CIDP 12 MMN 13 MADSAM 14 Optic neuritis 15 Neuroretinitis 16 Auditory neuropathy 17 Peripheral polyneuropathy 18 Neuromyelitis optica 19 Myeloradiculopathy 20 Myeloradiculoneuropathy 21 Hypokalemic paralysis 22 Encephalomyeloradiculopathy 99 n/a PNS_Dx_tfdo Time from disease onset is defined as: time from occurrence of first symptoms (fever, rash, arthralgia) to occurrence of neurological symptoms. The latter point in time can be described explicitly in the case report and can thus occur before hospitalization; if not described explicitly the day of hospitalization is used (description upon neurological examination). Example: Fever on Day 1. Lower limb weakness on day 16. Tfdo = 15.
99 n/a missing value Other_fever 0 no fever Code 1 if symptom is described in case report. 1 fever (≥38.0°C) Code 0 if symptom is not mentioned, but in general (other) initial 99 n/a symptoms are described. Other_rash 0 no rash Code 99 (missing value) if no initial symptoms are described at all. 1 rash 99 n/a Other_arthr 0 no arthralgia 1 arthralgia 99 n/a Other_syst 0 no other syst. sympt. 1 other syst. sympt. 99 n/a Clin_pres_consistent 0 Not consistent, or other DD more probable For consistency see CNS/PNS-Dx definitions 1 Clinical signs consistent with Dx Clin_pres_descr String Reason noted if inconsistency Etiol_exclud_descr String All etiologies excluded by authors are noted Etiol_exclud_suff 0 Frequent etiologies of neurological affection at According to sources noted under CNS_Dx and PNS_Dx stake not excluded Special rules: - in encephalitis: at least one common viral cause of encephalitis had to be excluded, while it was left to authors discretion which one are relevant in that respective area, season and clinical setting. - in GBS: C. jejuni was considered the most common causative agent, hence this one had to be excluded at least. 1 Other etiologies sufficiently excluded Blood_rtPCR 0 neg. 1 pos. 99 n/a missing value Blood_rtPCR_tfdo 99a n.s. time from occurrence of first symptoms (fever, rash, arthralgia) to specimen retrieval. Example: Fever on day 1. Serologic testing for IgM on day 5. Tfdo = 4 days. Blood_IgM 0 neg. Assumption: positive CHIK hemagglutination-inhibition (HI) antibody 1 pos. titer is going to be coded as “IgM positive, IgG negative“, as both immuneglobulines can account for HI-test positivity, but presence of IgM is more probable in the acute setting encountered in most case reports. 99 n/a missing value Blood_IgM_tfdo time from occurrence of first symptoms (fever, rash, arthralgia) to specimen retrieval. 99 n/a missing value Blood_IgG 0 neg. 1 pos. 99 n/a missing value Blood_IgG_tfdo time from occurrence of first symptoms (fever, rash, arthralgia) to specimen retrieval. 99 n/a missing value Blood_NeuroAb 0 neg. 1 pos. 99 n/a missing value CSF_rtPCR 0 neg. 1 pos. 99 n/a missing value CSF_rtPCR_tfdo time from occurrence of first symptoms (fever, rash, arthralgia) to specimen retrieval. 99 n/a missing value CSF_IgM 0 neg. 1 pos. 99 n/a missing value CSF_IgM_tfdo time from occurrence of first symptoms (fever, rash, arthralgia) to specimen retrieval. 99 n/a missing value CSF_IgG 0 neg. 1 pos. 99 n/a missing value CSF_IgG_tfdo time from occurrence of first symptoms (fever, rash, arthralgia) to specimen retrieval. 99 n/a missing value CSF_NeuroAb 0 neg. 1 pos. 99 n/a missing value CSF_basic 0 normal Cells < 5/µl, total protein 5 - 40mg/dl 1 exsudative CSF-Syndrome Cells > 100/µl, total protein slightly elevated* 2 transsudative CSF-Syndrome Cells 5-100/µl, total protein < 100mg/dl* 3 Guillain-Barré CSF-Syndrome Cells 0 - 50/µl, total protein ca. 100-200mg/dl ** (cytoalbuminologic dissociation) 4 Nonne-Froin CSF-Syndrome Cells < 5/µl, total protein >100-200mg/dl 5 Immunoactive CSF-Syndrome Cells 5 - 50/µl (evt. 5-100/µl), total protein < 80mg/dl 99 n/a missing value * greater significance is attached to cell count. Thus, f.ex. cells 68/µl & total protein 150mg/dl is considered transsudative, cells 160/µl & total protein 80mg/dl is considered exsudative. ** in 2nd week after disease onset; if CSF obtained earlier, total protein can be lower. In case blood is present and clinical history not suggestive of subarachnoidal hemorrhage a traumatic tap is assumed and the following rules are applied: 1 white blood cell subtracted for every 500rbc, 10mg/dl protein subtracted for every 1'000 rbc Sources for CSF-syndromes: [19,11] CSF_wbc 99 n/a missing value CSF_totProt 99 n/a missing value CSF_basic_tfdo time from occurrence of first symptoms (fever, rash, arthralgia) to specimen retrieval. 99 n/a missing value Imaging 0 not obtained Assumption: If no imaging modality is mentioned, “not obtained“ is coded. 1 MRI 2 CT 3 US 99 n/a missing value Code this if unclear which imaging modality was used, but imaging study is mentioned. Imag_tfdo time from occurrence of first symptoms (fever, rash, arthralgia) to imaging study. 99 n/a missing value Imag_result 0 no finding 1 path. finding 99 n/a missing value EEG 0 not obtained Assumption: If no EEG result is mentioned, „not obtained“ is coded. 1 obtained EEG_tfdo time from occurrence of first symptoms (fever, rash, arthralgia) to EEG study. 99 n/a missing value EEG_result 0 no finding 1 focal changes 2 diffuse slowing 3 pathologic but n.s. 99 n/a missing value ENMG / other NCS 0 not obtained Assumption: If no ENMG result is indicated, „not obtained“ is coded. Visual evoked potentials (VEP), Auditory evoked potentials (AEP, BERA etc.) are considered as NCS. 1 obtained ENMG_tfdo time from occurrence of first symptoms (fever, rash, arthralgia) to ENMG study. 99 n/a missing value ENMG_result 0 no finding 1 axonal damage 2 demyelinating 3 combined 4 pathologic but n.s. 99 n/a missing value Therapy 0 conservative 1 CTC 2 ivIg 3 PlasmaEx 4 CYC 5 CTC + PlasmaEx 6 CTC + ivIg 99 n/a missing value Therapy_tfdo time from occurrence of first symptoms (fever, rash, arthralgia) to onset of therapy 99 n/a missing value Therapy_tfNo time from occurrence of neurological symptoms to onset of therapy 99 n/a missing value Therapy_resp 0 no response 1 partial response 2 good response 99 n/a PathMech_susp 0 unclear This item is coded based on previous items’ values: 1 direct neurotoxic - supportive for „direct neurotoxic“: short CNS_tfdo (<7d.), 2 immunologic serum/CSF rtPCR pos. and Immunoglobulins neg. 3 combined - supportive for „immunologic“: CNS_Dx= ADEM, Bickerstaff, GBS 99a n.s. spectrum, Miller-Fisher-Syndrome, Neuromyelitis optica, CNS/PNS_tfdo >7-14 d., serum/CSF rtPCR neg. and/or Immunoglobulins positive, detection of antibodies associated with presumably neuroimmunological disorders (such as Anti- GQ1b, -GT1a, -NMO etc.) - “unclear“ coded if evidence points in both directions - “combined“ coded if biphasic disease pattern with first neurotoxic and secondly immunologic affection PathMech_ther 0 direct neurotoxic This item is coded based on previous item “therapy“: 1 immunologic - conservative treatment -> “direct neurotoxic“ - all kinds of immunomodulating treatment -> “immunologic“ 99 n/a missing value ICU_compl_present 0 present No complications requiring admission to intensive care unit (ICU) 1 present complications requiring admission to intensive care unit (ICU) 99 n/a Code 99 if complications requiring ICU-Support possible or probable given nature of main diagnosis, but not exactly stated in case report. ICU_Chall_biggest 0 none Code „none“ if stated so in case report or if very probably no (most severe complications requiring ICU-Support given nature of main diagnosis. complication present) 1 altered mental status If multiple complications reported (e.g. seizure=2 and respiratory 2 seizure failure=4), code the complication with the higher value (e.g.: 3 unconscious (GCS≤8), intubation 4=respiratory failure. 4 resp. failure, ventilation 5 cardiovascular (MI, HF, Arr., hemodynamic) 6 infection, SIRS, DIC, sepsis 7 intracranial hypertension 99 n/a Code 99 if complications requiring ICU-Support possible or probable given nature of main diagnosis, but not exactly stated in case report. AMS 0 no 1 yes 99 n/a missing value Seizure 0 no 1 yes 99 n/a missing value UC_Intub 0 no 1 yes 99 n/a missing value Vent 0 no 1 yes 99 n/a missing value CardioV 0 no 1 yes 99 n/a missing value InfSiSe 0 no 1 yes 99 n/a missing value IntraHT 0 no 1 yes 99 n/a missing value ICU_compl_severe 0 no complications No complications requiring admission to intensive care unit (ICU) (semiquantitative scale of severity) 1 moderate complications Moderate complications (ICU-Chall_biggest 1-3) 2 severe complications severe complications (ICU-Chall_biggest 4-7) 99 n/a missing value Outcome_semiquant 0 no symptoms, full recovery Due to variable ways of reporting, point in time when outcome is 1 partial recovery assessed is defined as last patient contact, be it on hospital discharge or 2 no recovery at last follow-up. 3 dead In newborns (<1Mo.) and infants (1-12Mo.) without evident sequelae 99 n/a without neurodevelopmental follow up code 99, as outcome cannot be assessed in a meaningful way. Outcome_mRS 0 no symptoms Comments under “Outcome_semiquant“ apply to Outcome_mRS, too. 1 no significant disability Able to carry out all usual activities, despite some symptoms. 2 slight disability Able to look after own affairs without assistance, but unable to carry out all previous activities. In case of residual arthralgia, code 2 3 moderate disability Requires some help, but able to walk unassisted. 4 moderately severe disability Unable to attend to own bodily needs without assistance, and unable to walk unassisted. 5 severe disability Requires constant nursing care and attention, bedridden, incontinent. 6 dead 99 n/a missing value REFERENCES
1. Shamseer L, Moher D, Clarke M et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj 2015;349:g7647. 2. Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. Chikungunya: A re-emerging virus. The Lancet 2012;379:662-671. 3. Higgins J, Altman D (2011) Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S (eds) Cochrane handbook for systematic reviews of interventions, vol 4. John Wiley & Sons, 4. Venkatesan A, Tunkel AR, Bloch KC et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2013;57:1114-1128. 5. Robin S, Ramful D, Le Seach F, Jaffar-Bandjee MC, Rigou G, Alessandri JL. Neurologic manifestations of pediatric Chikungunya infection. Journal of Child Neurology 2008;23:1028-1035. 6. Fodor PA, Levin MJ, Weinberg A, Sandberg E, Sylman J, Tyler KL. Atypical herpes simplex virus encephalitis diagnosed by PCR amplification of viral DNA from CSF. Neurology 1998;51:554-559. 7. Wakerley BR, Uncini A, Yuki N, Group GBSC, Group GBSC. Guillain-Barre and Miller Fisher syndromes--new diagnostic classification. Nature reviews Neurology 2014;10:537-544. 8. Storch-Hagenlocher B, Wildemann B, Berlit P, Kramer M (2015) Autoimmunerkrankungen. In: Schwab S, Schellinger P, Werner C, Unterberg A, Hacke A (eds) NeuroIntensiv, vol 3. Springer, Berlin Heidelberg, pp 577-608 9. O'Riordan JI, Gallagher HL, Thompson AJ et al. Clinical, CSF, and MRI findings in Devic's neuromyelitis optica. Journal of neurology, neurosurgery, and psychiatry 1996;60:382-387. 10. Wakerley BR, Yuki N. Mimics and chameleons in Guillain-Barre and Miller Fisher syndromes. Practical neurology 2015;15:90-99. 11. Adelmann M, Bamborschke S, Becker D et al. (2004) Leitlinien der Liquordiagnostik der DGLN 12. Maurer M, Wessig C, Kiefer R, Sommer C (2012) Immunneuropathien. In: Stangel M, Maurer M (eds) Autoimmunerkrankungen in der Neurologie. Springer, pp 115-152 13. Wakerley BR, Yuki N. Pharyngeal-cervical-brachial variant of Guillain-Barre syndrome. Journal of neurology, neurosurgery, and psychiatry 2014;85:339-344. 14. Bourque PR, Chardon JW, Massie R. Autoimmune peripheral neuropathies. Clinica chimica acta; international journal of clinical chemistry 2015;449:37-42. 15. Wakerley BR, Yuki N. Polyneuritis cranialis--subtype of Guillain-Barre syndrome? Nature reviews Neurology 2015b;11:664. 16. Wakerley BR, Yuki N. Polyneuritis cranialis: oculopharyngeal subtype of Guillain-Barre syndrome. Journal of neurology 2015a;262:2001-2012. 17. Bialasiewicz A. Neuroretinitis. Ophthalmologe 2000 97:374–391. 18. Garg RK, Malhotra HS, Verma R, Sharma P, Singh MK. Etiological spectrum of hypokalemic paralysis: A retrospective analysis of 29 patients. Ann Indian Acad Neurol 2013;16:365-370. 19. Delank H-W, Gehlen W (2006) Neurologie. Georg-Thieme Verlag.