Chemistry Midterm Study Guide Spring 2012 1. What types of properties are dependent upon the particles in a solution? 2. What is the % composition of oxygen in CO2? 3. Which part of the solution (solute or solvent) is water in an aqueous solution? 4. What is a precipitate? 5. Name and describe three types of mixtures. 6. Could you add more sugar to an unsaturated Kool Aid solution? 7. What type of chemical reaction produces heat? 8. In the following chemical reaction, how many moles of oxygen are needed to produce 10 moles of water? 2 H2 + 1 O2 2 H2O 9. If salt is added to water, will the electrical conductivity of the water increase or decrease? 10. What three factors increase the solubility of a solid? 11. Define the term solvation. 12. As solute is added to a solution, what happens to the boiling point, melting point, vapor pressure, and the osmotic pressure of the solution? 13. What is the solute in a sugar water solution? 14. If water and alcohol are miscible, what are two liquids that are immiscible? 15. What is the molarity of a solution containing 90 grams of C6H12O6 in 1 liter of water? 16. How many grams of H2O can be produced in the following reaction with 10 grams of oxygen? H2 + O2 H2O 17. What will polar solvents dissolve?
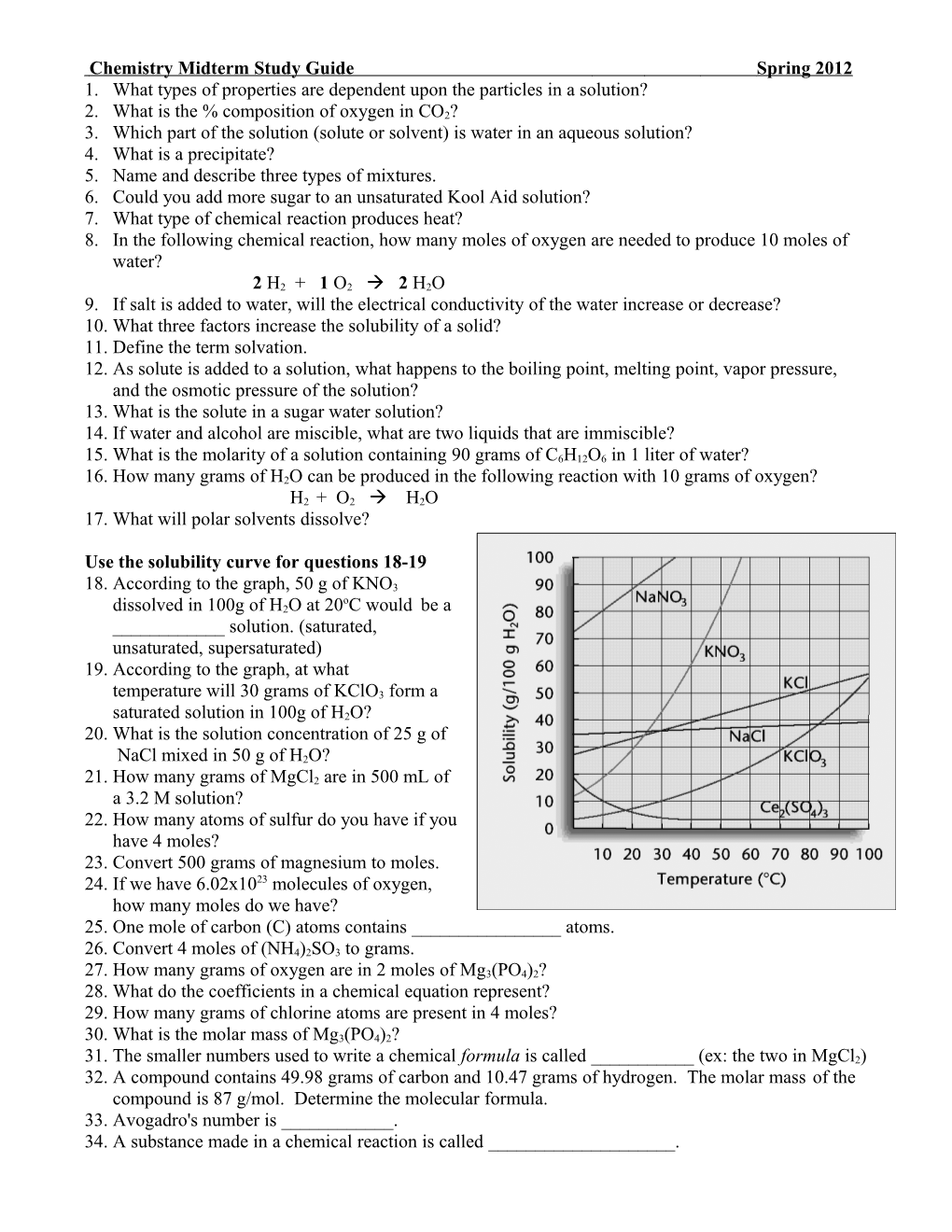
Use the solubility curve for questions 18-19 18. According to the graph, 50 g of KNO3 o dissolved in 100g of H2O at 20 C would be a ______solution. (saturated, unsaturated, supersaturated) 19. According to the graph, at what temperature will 30 grams of KClO3 form a saturated solution in 100g of H2O? 20. What is the solution concentration of 25 g of NaCl mixed in 50 g of H2O? 21. How many grams of MgCl2 are in 500 mL of a 3.2 M solution? 22. How many atoms of sulfur do you have if you have 4 moles? 23. Convert 500 grams of magnesium to moles. 24. If we have 6.02x1023 molecules of oxygen, how many moles do we have? 25. One mole of carbon (C) atoms contains ______atoms. 26. Convert 4 moles of (NH4)2SO3 to grams. 27. How many grams of oxygen are in 2 moles of Mg3(PO4)2? 28. What do the coefficients in a chemical equation represent? 29. How many grams of chlorine atoms are present in 4 moles? 30. What is the molar mass of Mg3(PO4)2? 31. The smaller numbers used to write a chemical formula is called ______(ex: the two in MgCl2) 32. A compound contains 49.98 grams of carbon and 10.47 grams of hydrogen. The molar mass of the compound is 87 g/mol. Determine the molecular formula. 33. Avogadro's number is ______. 34. A substance made in a chemical reaction is called ______. 35. What is the S.I. unit for each of the following? Mass ______Molar Mass ______Volume ______Molarity ______36. Balance the following reactions: Ca + H2O Ca(OH)2 + H2 P4O10 + H2O H3PO4 Fe + Cl2 FeCl3 CH4 + O2 CO2 + H2O Fe + Ag(NO2)2 Fe(NO2)3 + Ag C6H12O6 + O2 CO2 + H2O Use the following information for questions 37 & 38: Ammonium nitrate (NH4NO3) is an important fertilizer and is also used in the manufacture of explosives and fireworks. It is produced by treating nitric acid (HNO3) with ammonia gas (NH3). The balanced equation for this reaction is: HNO3 + NH3 NH4NO3 37. If 6 moles of ammonia gas are used with 4 moles of nitric acid for the reaction, what is the limiting reactant? 38. How many moles of ammonium nitrate would you make from the ingredients in the problem above? 39. Define the following terms: a. reactant g. law of conservation of mass b. product h. stoichiometry c. limiting reactant i. mole d. excess reactant j. molar mass e. empirical formula k. molecular mass f. molecular formula 40. Match the correct definition to the formula that it represents; ____H2O2 a. empirical formula ____H2O b. molecular formula ____C6H12O6 c. hydrate ____CH2O ____CuSO4▪ 5H2O 41. How does the total mass of the reactants compare to the total mass of the products in a chemical reaction? This is true according to what law? (Hint: to satisfy this law we balance chemical reactions) 42. What is the % of carbon in CO2? 43. a. A 32.8 gram sample of a compound contains 2.00 g of carbon, 22.8 g of barium and 8.00 g of oxygen, what is its empirical formula? b. What is the empirical formula for monosodium glutamate (MSG) if it is composed of 35.5% C, 4.77% H, 8.29% N,13.6% Na and 37.9% O? 44. What is the molecular formula for a compound with an empirical formula of NO2 and a molecular mass of 92.0 g? 44. The following equation shows the formation of aluminum oxide: 4 Al + 3 O2 --> 2 Al2O3 How many grams of aluminum are needed to produce 250 g of aluminum oxide? 45. Identify the type of bond in each of the following (ionic, polar covalent, non-polar covalent): NaCl CO HCl H2O 46. If 0.55 g of a gas dissolves in 1.0 L of water at 20.0 atm of pressure, how much will dissolve at 110.0 atm of pressure? 48. In the reaction between calcium hydride and water, the theoretical yield of hydrogen gas from 75.0 grams of calcium hydride is 7.18 grams. In running this reaction, the actual amount of hydrogen produced was 6.94 grams. What was the percent yield of this reaction? Answers: 1. Colligative 2. 72.7% 3. Solvent 4. Solid that settles to the bottom in a chemical reaction 5. Solutions (clear, homogeneous, cannot be filtered, do not exhibit the Tyndall Effect); Colloid (cloudy, heterogeneous, cannot be filtered, do exhibit the Tyndall Effect); Suspensions (cloudy, heterogeneous, can be filtered, do exhibit the Tyndall Effect) 6. Yes 7. Exothermic 8. 5 moles 9. Increase 10. Increase temperature, agitate, decrease particle size 11. Process of a solute breaking apart in a solvent 12. B.P. Increases, M.P. decreases, V. P. Decreases, O.P. Decreases 13. The sugar 14. Oil and water 15. .5 M 16. 11.25 g 17. Polar and ionic solutes 18. Supersaturated 19. 71°C 20. 33% 21. 150.4 g 22. 2.408x1024 atoms 23. 20.83 moles 24. 1 mole 25. 6.02x1023 atoms 26. 464 g 27. 16 g 28. Number of moles of each substance 29. 280 g 30. 264 g/mol 31. subscripts 32. C6H15 33. 6.02x1023 34. product 35. grams; cm3 or liters; grams/mole; moles/liter 36. a. 1,4,1,1 b.1,6,4 c. 2,3,2 d.1,2,1,2 e. 2,3,2,3 f. 1,6,6,6 37. HNO3 38. 4 mol 39. a. left of the yield sign b. formed in a chemical reaction c. reactant that is used up in a chemical reaction, limits the amount of product produced d. reactat left over after a chemical reaction is over e. reduced chemical formula f. How chemicals actually bond to make a molecule or formula unit g. the total mass or energy of the reactants = the mass or energy of the products h. quantitative analysis to calculate the relationships of reactants and products in a chemical reaction i. unit for the number of particles in a substance, Avogadro’s number, 6.02 x 1023 j. the mass of 1 mole of a substance in grams k. the mass of a molecule in atomic mass units 40. b, a and b, b, a, c 41. equal, Law of Conservation of Mass 42. 27% 43.BaCO3 44. N2O4 45. 132 grams Al 46. 3.0 g/L 48. 96.7%