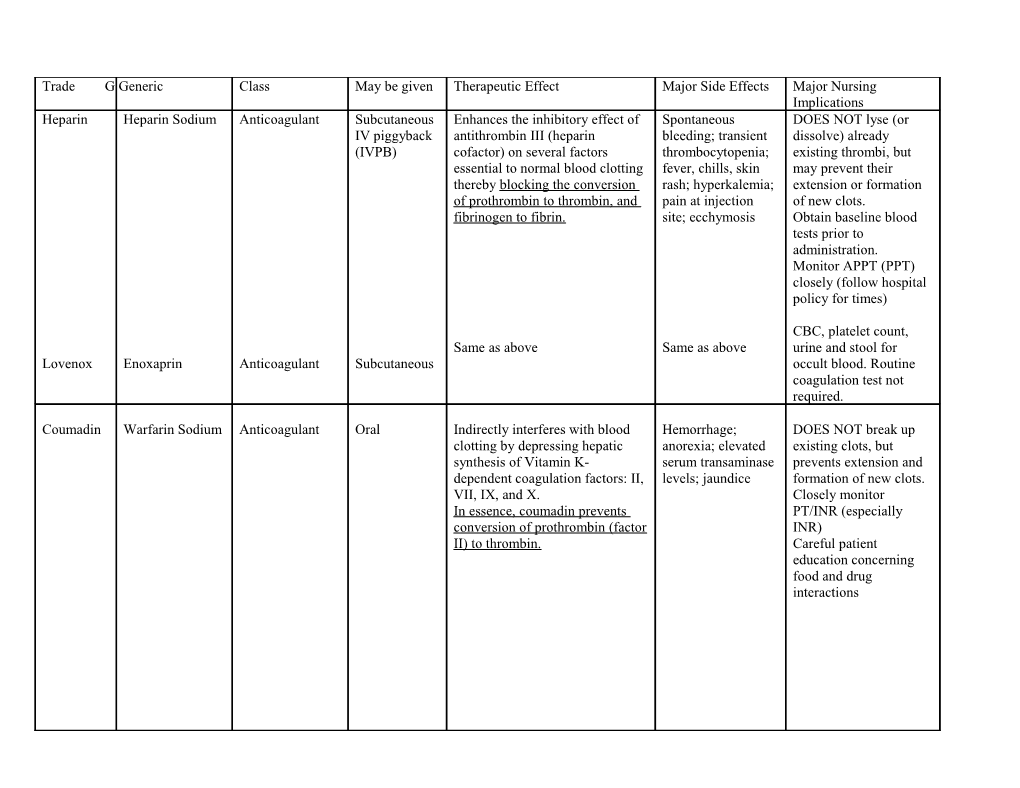
Trade G Generic Class May be given Therapeutic Effect Major Side Effects Major Nursing Implications Heparin Heparin Sodium Anticoagulant Subcutaneous Enhances the inhibitory effect of Spontaneous DOES NOT lyse (or IV piggyback antithrombin III (heparin bleeding; transient dissolve) already (IVPB) cofactor) on several factors thrombocytopenia; existing thrombi, but essential to normal blood clotting fever, chills, skin may prevent their thereby blocking the conversion rash; hyperkalemia; extension or formation of prothrombin to thrombin, and pain at injection of new clots. fibrinogen to fibrin. site; ecchymosis Obtain baseline blood tests prior to administration. Monitor APPT (PPT) closely (follow hospital policy for times)
CBC, platelet count, Same as above Same as above urine and stool for Lovenox Enoxaprin Anticoagulant Subcutaneous occult blood. Routine coagulation test not required.
Coumadin Warfarin Sodium Anticoagulant Oral Indirectly interferes with blood Hemorrhage; DOES NOT break up clotting by depressing hepatic anorexia; elevated existing clots, but synthesis of Vitamin K- serum transaminase prevents extension and dependent coagulation factors: II, levels; jaundice formation of new clots. VII, IX, and X. Closely monitor In essence, coumadin prevents PT/INR (especially conversion of prothrombin (factor INR) II) to thrombin. Careful patient education concerning food and drug interactions Plavix Clopidrogrel Antithrombotic oral Inhibits platelet aggregation by Flu-like symptoms; Monitor for and Bisulfate selectively preventing the binding epistaxis; immediately report Platelet of adenosine diphosphate to its thrombotic S/S of GI bleed. aggregation platelet receptor, and the thrombocytopenic Monitor platelet count inhibitor subsequent ADP-mediated purpura; rash and lipid profile. activation of the glycoprotein Evaluate patients with GPIIb/IIIa complex. This results in unexplained fever or prolonged bleeding time. infections for Clopidogrel also inhibits platelet myelotoxicity. aggregation induced by agonists other than ADP by blocking the amplification of platelet activation by released ADP. Clopidogrel does not inhibit phosphodiesterase activity. Clopidogrel acts by irreversibly modifying the platelet ADP receptor. Consequently, platelets exposed to clopidogrel are affected for the remainder of their lifespan.
Aspirin Acetylsalicytic Platelet oral Inhibition of platelet aggregation is Bronchospasm; Assess for S/S acid aggregation attributable to the inhibition of tinnitus; nausea, toxicity, especially in inhibitor platelet synthesis of thromboxane heartburn, GI bleed; older adults. A2, a potent vasoconstrictor and thrombocytopenia Monitor hepatic and inducer of platelet aggregation. renal function. This effect occurs at low doses and lasts for the life of the platelet (8 days). Higher doses inhibit the synthesis of prostacyclin, a potent vasodilator and inhibitor of platelet aggregation. Type 2 Diabetes Drugs Drug Classification Therapeutic Action Trade Name Generic Name Onset Dose Ranges Sulfonylurea (2nd Promote increased secretion Amaryl Glimepiride ~ 1 hr. Peaks in 1-2 hrs Initially 1-2 mg once daily generation) of insulin by the pancreas with breakfast. Maintenance dose 1-4 mg. once daily. After dose of 2 mg. is reached, increase in increments of 1-2 mg. at 1- 2 wk. intervals based on glucose levels. Maximum dose is 8 mg. once daily. Glucotrol Glipizide ~1-1.5 hr. Duration 10-16 Initially, 5 mg daily in a hr single dose before breakfast. In elderly, may start with 2.5 mg. Maximum dose 40 mg daily DiaBeta Glyburide 2-4 hr. Duration 24 hr. Initially 2.5-5 mg daily in a Micronase Glynase is better single dose. Maximum Glynase Pres Tab absorbed, acts faster dose 20 mg daily. (onset 1 hr,), and is given Glynase initial dose 1.5-3 in smaller doses than other mg daily. Maximum glyburides dosage 12 mg daily. Alpha-Glucosidase Delay the digestion and Precose Acarbose Onset with ingestion. Peak Initially 25 mg, 3 times a Inhibtor absorption of complex ~70 min. after dose. Half- day with first bite of meals. carbohydrates into simple life 2 hrs. Maximum of 50 mg, 3 sugars when medications times a day. If patient and food are in the GI tract weight > 60 kg, maximum at the same time is 100 mg, 3 times a day with meals. Glyset Miglitol Onset with ingestion. Peak Initially 25 mg, 3 times a 2-3 hr after dose. Half-life day with first bite of meals. 2 hr Maximum dosage 100 mg, 3 times a day with meals Biguanide Improves tissue sensitivity to Glucophage Metformin Onset of action is Initially 500mg daily, insulin; increases insulin slow. Satisfactory control advancing to 500 mg, 3 absorption and transport of of blood glucose times a day if needed, or to glucose into skeletal muscle concentration may occur 850mg every twelve hours and fat; suppresses within a few days to a (with or after food). gluconeogenesis and hepatic week of initiating therapy, Maximum of 3g daily in production of glucose; although the maximum divided doses although this decreases intestinal anti-hyperglycemic effect is normally limited to 2g reabsorption of glucose (reduction in blood sugar daily. Do not increase levels) may be delayed for elderly patients to up to two weeks maximum dose.
Thiazolidinedione; Decreases insulin resistance Actos Pioglitozone Onset with ingestion. Peak 15-30 mg. once daily Glitazone in skeletal muscles and 2 hr. Duration 24 hr. adipose tissue and decrease Avandia Rosiglitazone Onset with ingestion. Peak 4-8 mg. once daily in one hepatic glucose production 1 hr. Food delays peak by dose or two divided doses. and output. 1.75 hr. Duration >24 hr. Meglitinide Stimulates release of insulin Starlix Nateglinide Rapidly absorbed. Peak 1 60-120 mg, 3 times a day from pancreatic cells hr. Half-life 1.5 hrs. taken 10 minutes before meals. Omit dose if meal not taken. Add a dose if extra meal is eaten. Never double dose. Prandin Repaglinide Rapidly absorbed. Peak 1 0.5-2 mg, 3 times daily 15 hr. Half-life 1 hr. minutes prior to meals. May increase to 4 mg. Omit dose if meal not eaten, add dose if extra meal eaten. Maximum dosage 16mg per day. Combination Drug Refer to information for each Glucovance Glyburide/metformin See table for individual See table for individual drug contained in ingredients ingredients combination product. Carefully check dosage. Medication is produced in varying strengths. Metaglip Metformin/glipizide
Carefully check dosage. Medication is produced in varying strengths. Avandamet Rosiglitazone/metformin
Carefully check dosage. Medication is produced in varying strengths. Maximum daily dose is 8 mg Rosiglitazone and 1000 mg metformin Incretin Mimetic Mimics the effect of naturally Byetta Exenatide Exenatide's absorption Two prefilled pens occurring hormones from the reaches median plasma (5 mcg or 10 mcg) are Injection only intestines, and can help the concentration 2.1 hours after available, depending on body make more of its own subcutaneous injection. The your prescribed dose (5 insulinExenatide has been drug is primarily eliminated shown to reduce gastric by glomerular filtration mcg or 10 mcg, emptying time, which in turn followed by proteolytic twice a day). Each pen has may affect the rate of degradation, with an 60 doses to provide 30 absorption of oral medications. elimination half-life of 2.4 days of twice–a–day Oral medications that are hours. injections. dependent on gastric emptying for bioavailability should not be given within 1 hour of exenatide injections. Amylin A synthetic form of the Symlin Pramlinide Pramlintide has been Never mix SYMLIN and hormone amylin, which is approved for people with type insulin. You must use Injection only produced along with insulin by 1 diabetes who are not different syringes for the beta cells in the pancreas. achieving their goal A1C SYMLIN and insulin because Amylin, insulin, and another levels and for people with insulin can affect SYMLIN hormone, glucagon, work in an type 2 diabetes who are using when the two are mixed interrelated fashion to maintain insulin and are not achieving together. Use a U-100 insulin normal blood glucose levels. their A1C goals. syringe (best to use 0.3 mL Pramlintide injections taken [0.3 cc] size) to draw-up and with meals have been shown inject SYMLIN to modestly improve A1C levels without causing Find Your Draw Up increased hypoglycemia or Dose in This weight gain and even micrograms Amount promoting modest weight (mcg) in U-100 loss. The primary side effect Insulin is nausea, which tends to Syringe improve over time and as an (units) individual patient determines 15 2½ his or her optimal dose. 30 5 45 7½ 60 10 120 20 Insulin Preparations Rapid Acting Short Acting Intermediate Acting Long Acting Combination Preparations Brand Name Humalog® Novolog® Novolin® R/ Humulin® N/ Humulin® L/ Humulin® U Lantus® Humulin/ Humulin Humalog (generic name) (insulin (insulin Humulin® R Novolin® N Novolin® L (insulin zinc (insulin Novolin 50/502 Mix lispro) aspart) (regular insulin) (NPH) (Lente®, insulin suspension, glargine) 70/301 75/253 zinc suspension) extended) Onset 0.25 h 0.5 h 1-2 h 1-3 h 4-6 h 1 h 0.5 h 0.25 h Peak 0.5-1.5 h 2-4 h 6-12 h 8-20 h N/A 2-12 h Duration 4-6 h 6-8 h 18-24 h 24-48 h 24 h 18-24 h Compatibility NPH, NPH All Regular Regular, Regular, NONE Regular NPH, Humulin® U Humulin® U Humulin® U Humulin® L *Use *Draw Novolin®/ * Draw Regular *Do NOT *Draw Humalog® *Use within within 15 Humulin® R into insulin into syringe *Lente® and *Humulin® U mix with any Mix 75/25 into 15 minutes of minutes of syringe before first to prevent Humulin® U may and Lente® other insulin syringe before mixing mixing longer acting clouding of R be mixed in any may be mixed preparations NPH or Humulin® insulins to prevent remaining in vial ratio in any ratio U to prevent * Draw *Draw clouding of R contaminating the Humalog® Novolog® remaining in vial *Regular and *Regular and insulin remaining into syringe into syringe Lente® mixtures Humulin® U in the Humulin® first when first when must be injected mixtures must Mix vial mixing mixing within 5 minutes be injected of mixing to within 5 **Inject prevent minutes of immediately after diminishing effect mixing to mixing. Do NOT of the regular prevent allow mixture to insulin diminishing remain unused in effect of the syringe regular insulin Comments -Administer immediately -Administer 30-60 -Roll vial between -Roll vial between -Roll vial -Should be a -Combination -Administer before meals minutes before hands prior to hands prior to between clear solution product are immediately before -Should be a clear solution meals drawing up to drawing up to hands prior to stable and are meals ensure adequate ensure adequate drawing up to -Lantus®: absorbed as if -Humalog®: pregnancy -Should be a clear dispersion dispersion ensure pregnancy injected -Humalog® Mix category B solution adequate category C separately 75/25 has a similar -Avoid shaking to -Avoid shaking to dispersion glucose lowering -Novolog®: pregnancy prevent formation prevent formation -Administer effect as compared category C of bubbles or foam of bubbles or foam -Avoid once daily at with 70/30 on a shaking to bedtime unit per unit basis; -Humalog® products are prevent however, the onset therapeutically interchanged formation of of 75/25 is more to Novolog® at AGMC bubbles or rapid. (Humalog® is utilized in foam pregnancy) 1 Contains 70% NPH insulin and 30% regular insulin 2 Contains 50% NPH insulin and 50% regular insulin 3 Contains 75% insulin lispro protamine suspension and 25% insulin lispro Rapid Acting Short Acting Intermediate Acting Long Acting New Product (NOT Insulin) Brand Name Exubera Apidra Levemir Symlin (Pramlintide) Onset 30 min 15 min 1 hr Peak 2 hrs. 20 min 1 hr none Duration 6 hrs. 30 min 1 hr 40 min 24 hours Compatibility Not compatible with If mixed with NPH, Should NOT be mixed with NONE cigarette smoking. Apidra should be drawn other insulins Because of differences in Increases incidence of up first. Injection chemistry, pramlintide hypoglycemic events. should be made cannot be combined in the IMMEDIATELY after same vial or syringe with mixing insulin and must be NOT compatible with injected separately other insulins Comments A dry powder Should only be used in Dispensed in a ‘pen’ injector. A synthetic form of the containing human regimens which include Patients must be instructed in hormone amylin, which is insulin, mannitol, a longer acting insulin proper use of injector and produced along with glycine, & sodium or basal insulin analog return demonstration to insulin by the beta cells in citrate. Stable at room assure proper dosage is the pancreas. Amylin, temp May be infused via obtained. insulin, and another insulin pump. Apidra hormone, glucagon, work Packaged in must be full changed in in an interrelated fashion individual dose- reservoir and tubing to maintain normal blood packs that include every 48 hours if used glucose levels. either 1 or 3 mg of in pumps. Pramlintide has been recombinant insulin approved for people with in powder form. Not safe for IV type 1 diabetes who are Each mg is administration not achieving their goal equivalent to 2 to 3 A1C levels and for people units of with type 2 diabetes who subcutaneous insulin are using insulin and are not achieving their A1C Should not be used in goals. patients with lung Pramlintide injections disease or smokers or taken with meals have those who stopped been shown to modestly smoking less than 6 improve A1C levels months ago without causing increased hypoglycemia or weight Prior to therapy, must gain and even promoting have pulmonary modest weight loss. The functions testing, and primary side effect is repeat testing every 6 nausea, which tends to months improve over time and as an individual patient determines his or her optimal dose.