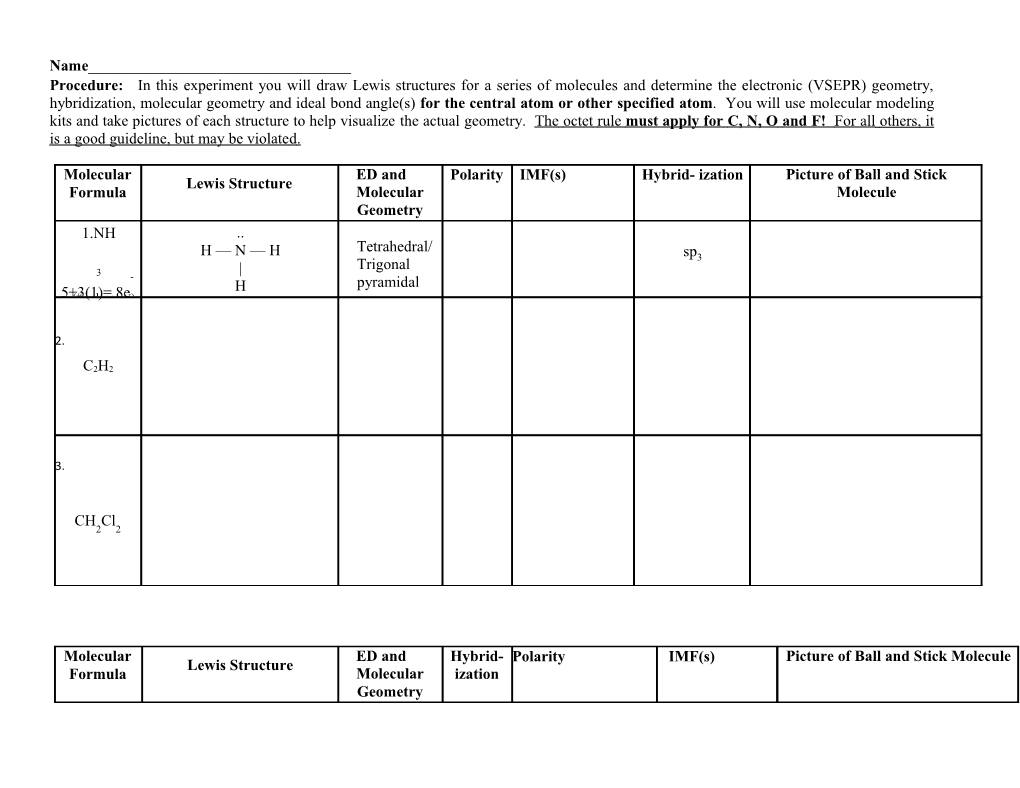
Name______Procedure: In this experiment you will draw Lewis structures for a series of molecules and determine the electronic (VSEPR) geometry, hybridization, molecular geometry and ideal bond angle(s) for the central atom or other specified atom. You will use molecular modeling kits and take pictures of each structure to help visualize the actual geometry. The octet rule must apply for C, N, O and F! For all others, it is a good guideline, but may be violate d .
Molecular ED and Polarity IMF(s) Hybrid- ization Picture of Ball and Stick Lewis Structure Formula Molecular Molecule Geometry 1.NH .. Tetrahedral/ H — N — H sp3 | Trigonal 3 - H pyramidal 5+3(1)=(# valence 8e e-)
2.
C2H2
3.
CH Cl 2 2
Molecular ED and Hybrid- Polarity IMF(s) Picture of Ball and Stick Molecule Lewis Structure Formula Molecular ization Geometry RnF 4
OF 6
CH NH 3 2
BBr 3 Molecular ED and Molecular Hybrid- Polarity IM Ideal Bond Picture of Ball and Stick Molecule Lewis Structure Formula Geometry ization F(s Angles )
SCl 2
AtF 3
SeF 4
BrF 5
3 Molecular ED and Molecular Hybrid- Polarity IM Ideal Bond Picture of Ball and Stick Molecule Lewis Structure Formula Geometry ization F(s Angles )
PCl 5
CH O 2
C H O 2 6 (CH OCH 3 3)
C H O 2 6 (C H OH) 2 5 Molecular ED and Molecular Hybrid- Polarity IM Ideal Bond Picture of Ball and Stick Molecule Lewis Structure Formula Geometry ization F(s Angles )
-1 ClO3
BeCl 2
NO -1 3
BH3
5 Molecular ED and Molecular Hybrid- Polarity IM Ideal Bond Picture of Ball and Stick Molecule Lewis Structure Formula Geometry ization F(s Angles )
H2O
CO 2
CH3CH2OH
2- CO3 1. From the Lewis Structures drawn in the lab, which substance(s) showed resonance.
2. Which substance from this acitivity would have the strongest London Dispersion force. Name three reasons why iyou selected this substance your explanation
3. In the space below, draw two water molecules hydrogen bonding with each other.
4. In the space below, choose another substance from this lab capable of hydrogen bonding, and draw hydrogen bonding with water
5. Choose the two substances from this activity who are likely to have very low boiling points. In terms of their intermolecular forcaes, explain why you chose them.
7