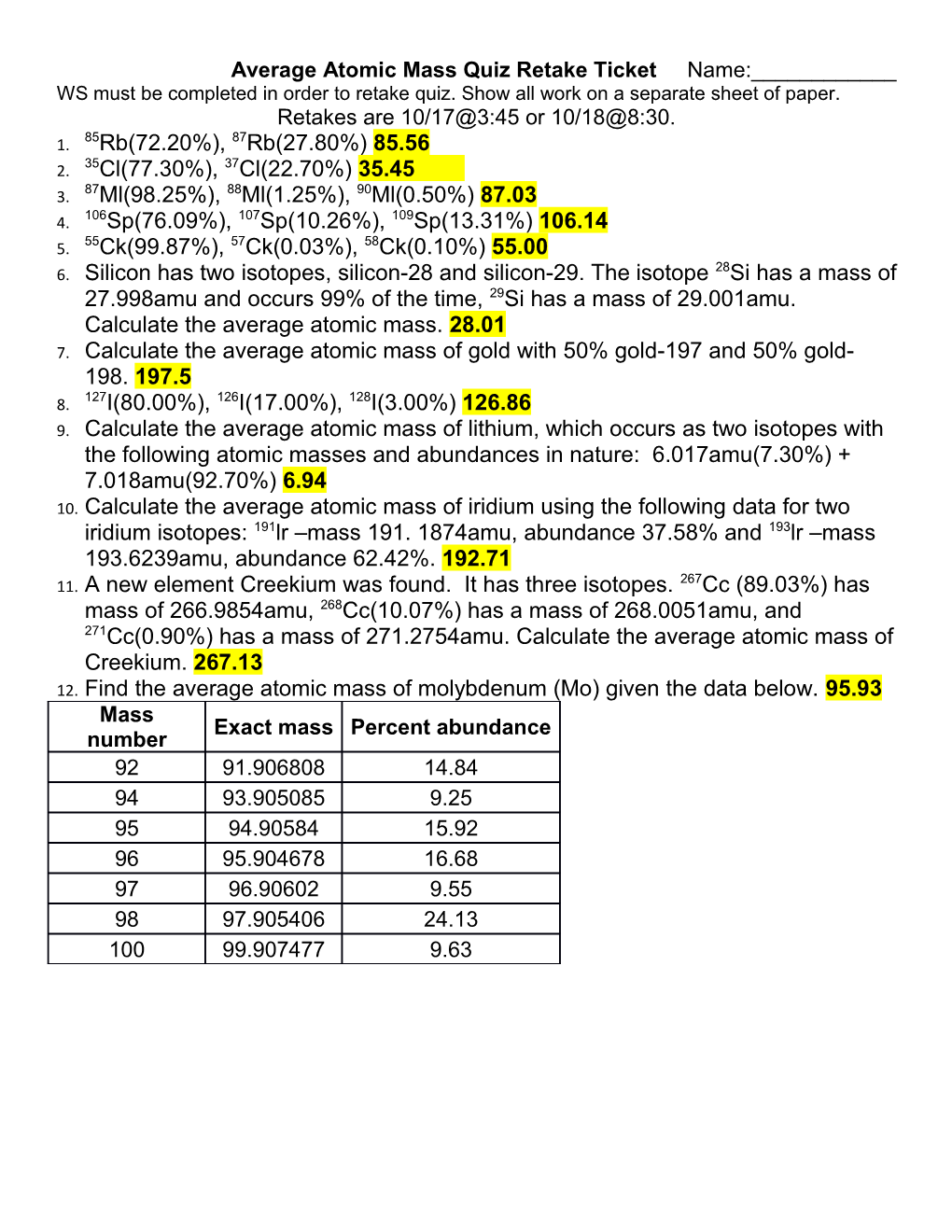
Average Atomic Mass Quiz Retake Ticket Name:______WS must be completed in order to retake quiz. Show all work on a separate sheet of paper. Retakes are 10/17@3:45 or 10/18@8:30. 85 87 1. Rb(72.20%), Rb(27.80%) 85.56 35 37 2. Cl(77.30%), Cl(22.70%) 35.45 87 88 90 3. Ml(98.25%), Ml(1.25%), Ml(0.50%) 87.03 106 107 109 4. Sp(76.09%), Sp(10.26%), Sp(13.31%) 106.14 55 57 58 5. Ck(99.87%), Ck(0.03%), Ck(0.10%) 55.00 28 6. Silicon has two isotopes, silicon-28 and silicon-29. The isotope Si has a mass of 27.998amu and occurs 99% of the time, 29Si has a mass of 29.001amu. Calculate the average atomic mass. 28.01 7. Calculate the average atomic mass of gold with 50% gold-197 and 50% gold- 198. 197.5 127 126 128 8. I(80.00%), I(17.00%), I(3.00%) 126.86 9. Calculate the average atomic mass of lithium, which occurs as two isotopes with the following atomic masses and abundances in nature: 6.017amu(7.30%) + 7.018amu(92.70%) 6.94 10. Calculate the average atomic mass of iridium using the following data for two iridium isotopes: 191lr –mass 191. 1874amu, abundance 37.58% and 193lr –mass 193.6239amu, abundance 62.42%. 192.71 267 11. A new element Creekium was found. It has three isotopes. Cc (89.03%) has mass of 266.9854amu, 268Cc(10.07%) has a mass of 268.0051amu, and 271Cc(0.90%) has a mass of 271.2754amu. Calculate the average atomic mass of Creekium. 267.13 12. Find the average atomic mass of molybdenum (Mo) given the data below. 95.93 Mass Exact mass Percent abundance number 92 91.906808 14.84 94 93.905085 9.25 95 94.90584 15.92 96 95.904678 16.68 97 96.90602 9.55 98 97.905406 24.13 100 99.907477 9.63 Average Atomic Mass Quiz Retake Ticket Name:______WS must be completed in order to retake quiz. Show all work on a separate sheet of paper. Retakes are 10/17@3:45 or 10/18@8:30. 85 87 1. Rb(72.20%), Rb(27.80%) 85.56 35 37 2. Cl(77.30%), Cl(22.70%) 35.45 87 88 90 3. Ml(98.25%), Ml(1.25%), Ml(0.50%) 87.03 106 107 109 4. Sp(76.09%), Sp(10.26%), Sp(13.31%) 106.14 55 57 58 5. Ck(99.87%), Ck(0.03%), Ck(0.10%) 55.00 28 6. Silicon has two isotopes, silicon-28 and silicon-29. The isotope Si has a mass of 27.998amu and occurs 99% of the time, 29Si has a mass of 29.001amu. Calculate the average atomic mass. 28.01 7. Calculate the average atomic mass of gold with 50% gold-197 and 50% gold- 198. 197.5 127 126 128 8. I(80.00%), I(17.00%), I(3.00%) 126.86 9. Calculate the average atomic mass of lithium, which occurs as two isotopes with the following atomic masses and abundances in nature: 6.017amu(7.30%) + 7.018amu(92.70%) 6.94 10. Calculate the average atomic mass of iridium using the following data for two iridium isotopes: 191lr –mass 191. 1874amu, abundance 37.58% and 193lr –mass 193.6239amu, abundance 62.42%. 192.71 267 11. A new element Creekium was found. It has three isotopes. Cc (89.03%) has mass of 266.9854amu, 268Cc(10.07%) has a mass of 268.0051amu, and 271Cc(0.90%) has a mass of 271.2754amu. Calculate the average atomic mass of Creekium. 267.13 12. Find the average atomic mass of molybdenum (Mo) given the data below. 95.93 Mass Exact mass Percent abundance number 92 91.906808 14.84 94 93.905085 9.25 95 94.90584 15.92 96 95.904678 16.68 97 96.90602 9.55 98 97.905406 24.13 100 99.907477 9.63
Average Atomic Mass Quiz Retake Ticket Name:______
Total Page:16
File Type:pdf, Size:1020Kb
Recommended publications