CHARGE OF AN ELECTRON – ELECTROLYSIS METHOD
INTRODUCTION: The object of this experiment is to determine the fundamental quantum charge, i.e. the magnitude of the charge possessed by an electron.
The method we will use employs a steady DC current to perform electrolysis of a CuSO4 solution. The current will be held constant for a known time interval, and the mass of copper plated on the cathode will be measured. Given the valence of copper ions we can then determine the charge per electron.
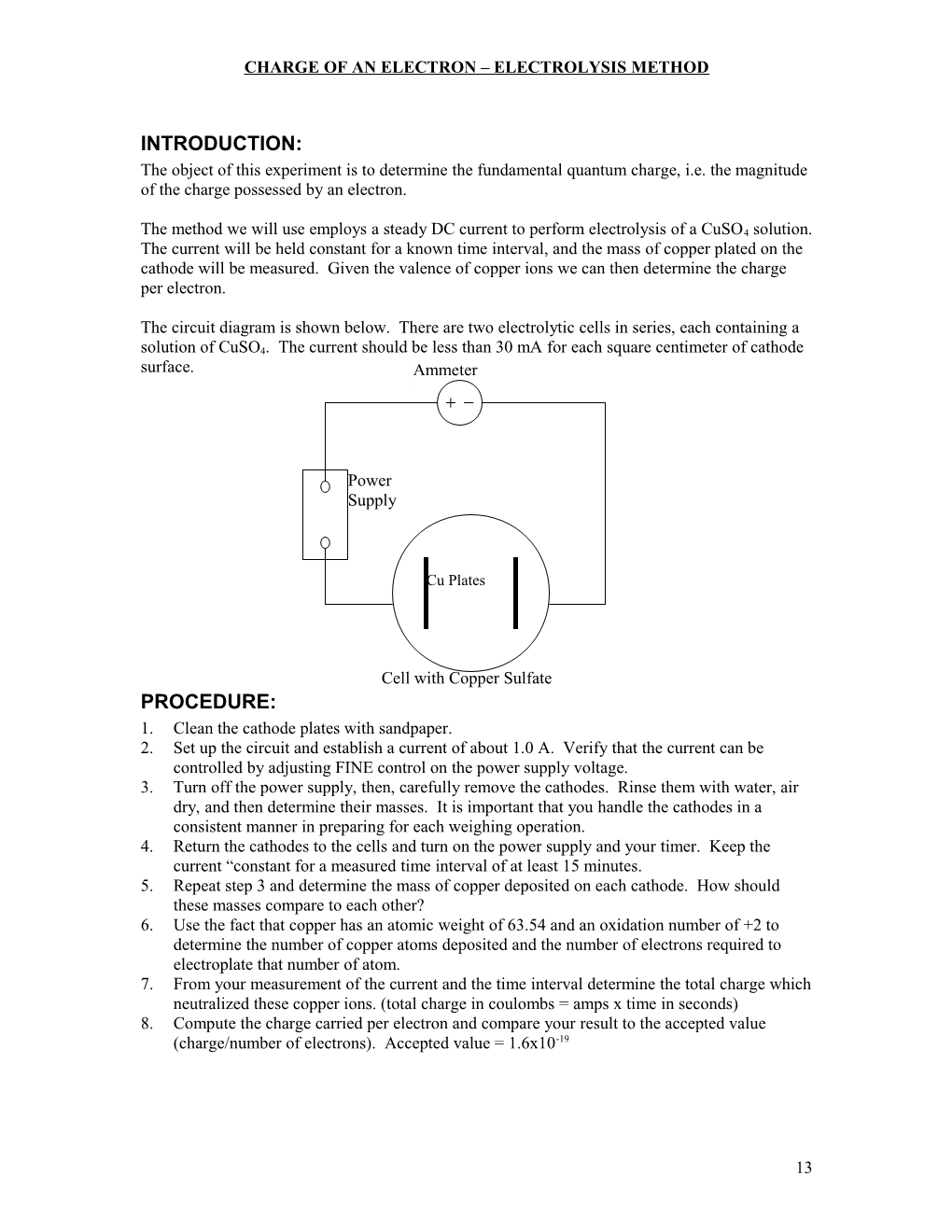
The circuit diagram is shown below. There are two electrolytic cells in series, each containing a solution of CuSO4. The current should be less than 30 mA for each square centimeter of cathode surface. Ammeter
Power Supply
Cu Plates
Cell with Copper Sulfate PROCEDURE: 1. Clean the cathode plates with sandpaper. 2. Set up the circuit and establish a current of about 1.0 A. Verify that the current can be controlled by adjusting FINE control on the power supply voltage. 3. Turn off the power supply, then, carefully remove the cathodes. Rinse them with water, air dry, and then determine their masses. It is important that you handle the cathodes in a consistent manner in preparing for each weighing operation. 4. Return the cathodes to the cells and turn on the power supply and your timer. Keep the current “constant for a measured time interval of at least 15 minutes. 5. Repeat step 3 and determine the mass of copper deposited on each cathode. How should these masses compare to each other? 6. Use the fact that copper has an atomic weight of 63.54 and an oxidation number of +2 to determine the number of copper atoms deposited and the number of electrons required to electroplate that number of atom. 7. From your measurement of the current and the time interval determine the total charge which neutralized these copper ions. (total charge in coulombs = amps x time in seconds) 8. Compute the charge carried per electron and compare your result to the accepted value (charge/number of electrons). Accepted value = 1.6x10-19
13