Acids and Bases Notes & Lab Activity
ACIDS The 3 properties of ACIDS are: 3 examples of common ACIDS are: 1. 1. 2. 2. 3. 3.
BASES The 3 properties of BASES are: 3 examples of common BASES are: 1. 1. 2. 2. 3. 3.
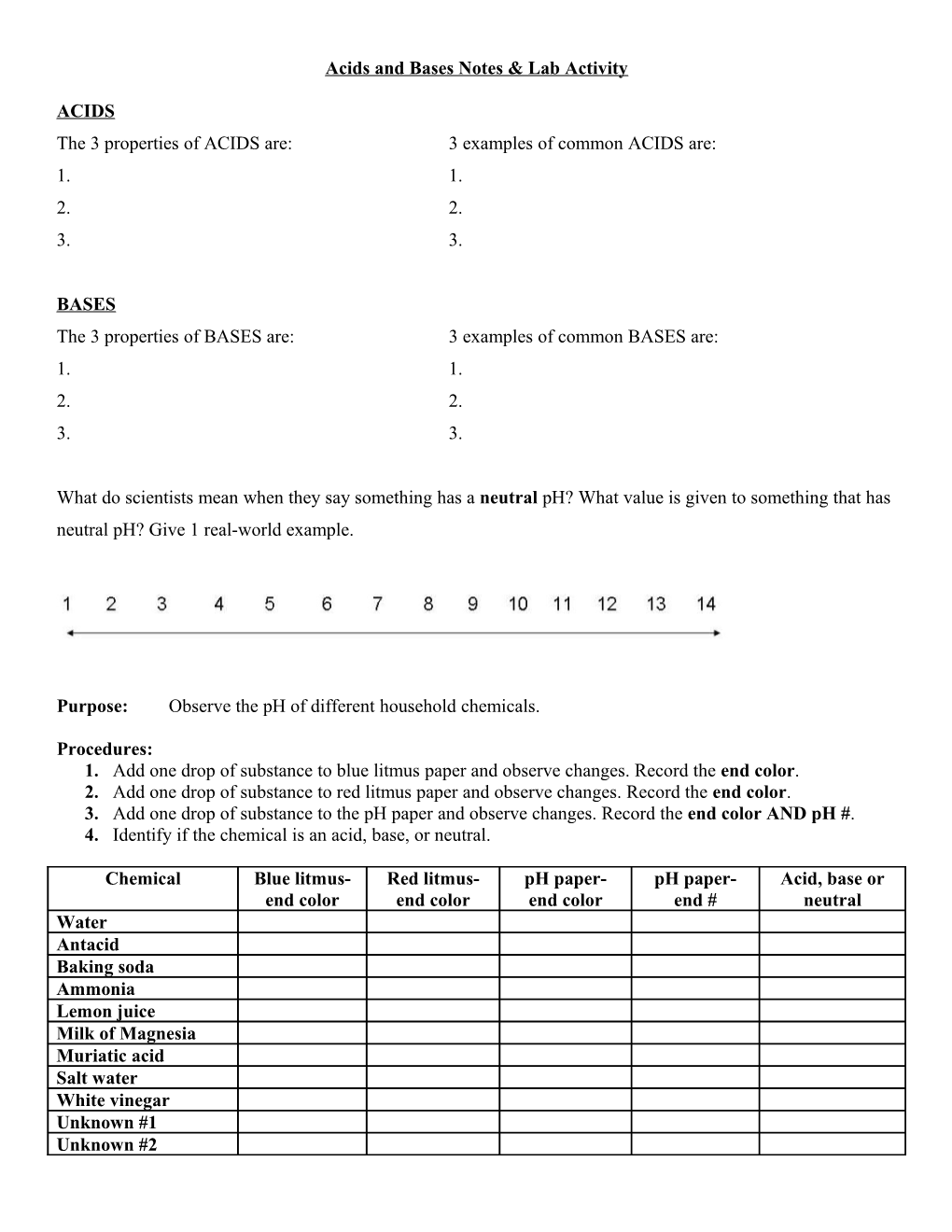
What do scientists mean when they say something has a neutral pH? What value is given to something that has neutral pH? Give 1 real-world example.
Purpose: Observe the pH of different household chemicals.
Procedures: 1. Add one drop of substance to blue litmus paper and observe changes. Record the end color. 2. Add one drop of substance to red litmus paper and observe changes. Record the end color. 3. Add one drop of substance to the pH paper and observe changes. Record the end color AND pH #. 4. Identify if the chemical is an acid, base, or neutral.
Chemical Blue litmus- Red litmus- pH paper- pH paper- Acid, base or end color end color end color end # neutral Water Antacid Baking soda Ammonia Lemon juice Milk of Magnesia Muriatic acid Salt water White vinegar Unknown #1 Unknown #2 Some household substances have warning labels on their containers; which substances do you think they were and WHY?
You are an environmental scientist and have been asked to research a local waterway and stream to determine why the fish in the area are dying. In addition there seems to be a lack of vegetation growing in the location around the stream.
You notice that there is a large industrial plant located up stream and the company is a major producer of sulfuric acid.
You collect a sample of water and begin to test the pH of the water in three locations (See below). The following results are what you discovered:
Location 1 Location 2 Location 3 At the end of the stream before A section of the stream 2 A location that runs right by it empties into a nearby lake miles away from the lake the industrial plant
pH 6 4 2
# of dead fish 4 7 27
1. Which location is the most acidic?
2. Draw a pH scale and label where each location would be located on the pH scale.
3. What can you conclude about Location 3 and its relationship to the industrial factory? (Be specific)
4. Use real logic and suggest a realistic way to neutralize the acidity in the stream?
5. What product will be produced after the neutralization?