1
Lab Handouts for CH342, Summer 2010
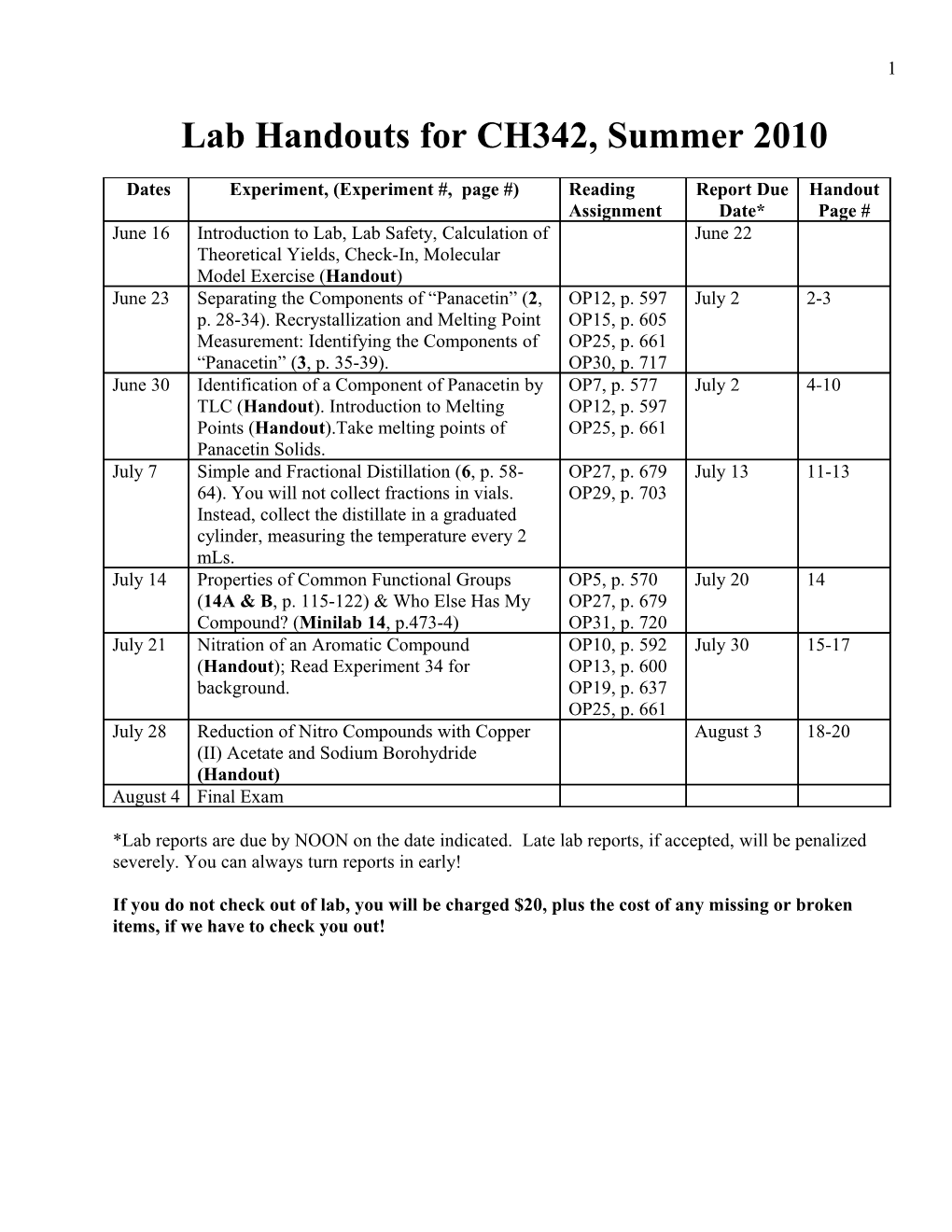
Dates Experiment, (Experiment #, page #) Reading Report Due Handout Assignment Date* Page # June 16 Introduction to Lab, Lab Safety, Calculation of June 22 Theoretical Yields, Check-In, Molecular Model Exercise (Handout) June 23 Separating the Components of “Panacetin” (2, OP12, p. 597 July 2 2-3 p. 28-34). Recrystallization and Melting Point OP15, p. 605 Measurement: Identifying the Components of OP25, p. 661 “Panacetin” (3, p. 35-39). OP30, p. 717 June 30 Identification of a Component of Panacetin by OP7, p. 577 July 2 4-10 TLC (Handout). Introduction to Melting OP12, p. 597 Points (Handout).Take melting points of OP25, p. 661 Panacetin Solids. July 7 Simple and Fractional Distillation (6, p. 58- OP27, p. 679 July 13 11-13 64). You will not collect fractions in vials. OP29, p. 703 Instead, collect the distillate in a graduated cylinder, measuring the temperature every 2 mLs. July 14 Properties of Common Functional Groups OP5, p. 570 July 20 14 (14A & B, p. 115-122) & Who Else Has My OP27, p. 679 Compound? (Minilab 14, p.473-4) OP31, p. 720 July 21 Nitration of an Aromatic Compound OP10, p. 592 July 30 15-17 (Handout); Read Experiment 34 for OP13, p. 600 background. OP19, p. 637 OP25, p. 661 July 28 Reduction of Nitro Compounds with Copper August 3 18-20 (II) Acetate and Sodium Borohydride (Handout) August 4 Final Exam
*Lab reports are due by NOON on the date indicated. Late lab reports, if accepted, will be penalized severely. You can always turn reports in early!
If you do not check out of lab, you will be charged $20, plus the cost of any missing or broken items, if we have to check you out! 2
Modifications to Panacetin Experiments 2 & 3
1. You will be given an unknown sample. Weigh it and use all of it.
2. For “Separation of Aspirin” on the middle of page 32, we will be using 5% sodium bicarbonate solution (NaHCO3), instead of 1M sodium hydroxide. Sodium hydroxide sometimes reacts with the ester group of aspirin.
3. For “Isolation of the Unknown Component” on the bottom of page 32, we will do the following:
a. Weigh your clean and dry 100 mL round- bottomed flask. b. Transfer your dichloromethane layer from the separation of Aspirin to the round-bottomed flask. c. Put a label on your cork ring with your name on it. d. Take your round-bottomed flask and the cork ring to the rotary evaporators in the middle of the lab, and have your dichloromethane removed. If the rotary evaporators are in use, leave your flask and ring there. e. When your dichloromethane has been removed, reweigh the flask to determine A Rotary Evaporator the mass of the unknown.
4. After you have isolated the unknown in step 3 above, scrape it out into a 125 mL Erlenmeyer flask. Any remaining unknown can be removed from the flask by rinsing the flask with a few mL of boiling water.
5. Allow your aspirin and unknown to dry thoroughly before you weigh them and determine their melting ranges. 3
Lab Report Format for Separating the Components of “Panacetin” (2) and Recrystallization and Melting Point Measurement: Identifying the Components of “Panacetin” (3).
1. Title Page
a. A descriptive title with between 15-25 words. b. Dates the experiment was performed. c. Course and section numbers. d. Your name e. Tape your TLC plates to the bottom of the page. Clearly label each plate and column of spots on the page so I know what it is.
2. Body of the report (start a new page)
a. Panacetin sample number. b. Mass of initial panacetin sample. c. Mass of sucrose collected. d. Mass of aspirin collected. e. Melting point range of aspirin. f. Mass of 100 mL round-bottomed flask. g. Mass of 100 mL round-bottomed flask plus unknown. h. Mass of unknown compound before recrystallization (g-f). i. Mass of unknown compound after recrystallization. j. Melting point range of recrystallized unknown compound. k. Mixture melting point ranges of the unknown compound with acetanilide and with phenacetin. l. Show your calculations of the percent recoveries of sucrose, aspirin and your unknown before recrystallization (divide the mass of each solid by answer in b., 100%). m. Calculate Rf values for all of the spots on your TLC plate. Show your work. n. What was the identity of your unknown? Explain in detail how you determined it, comparing the data you obtained with known values. Explain any discrepancies.
3. Questions
a. Suppose you left a small amount of the aqueous solution with the dichloromethane solution after you did the extractions, and the water evaporated with the dichloromethane solution on the rotovap. How would this affect the yield of your unknown? How would this affect the purity of your unknown? Explain your reasoning. b. Suppose you acidified the sodium bicarbonate solution to a pH of 5, rather than to a pH of 2. How would this affect the yield of your aspirin? How would this affect the purity of your aspirin? Explain your reasoning: what physical constant is important to know when you choose a pH to acidify to? c. What happened to the Rf values of your spots when you used the different TLC solvent? Why? Explain your reasoning. 4
Thin-Layer Chromatography (TLC) Of Recovered Panacetin Products
In this experiment, you will be assessing the purity of your crops of recrystallized Panacetin unknown, as well as your aspirin. Your unknown is either acetanilide or phenacetin. Since aspirin, acetanilide, and phenacetin show up under UV light, we can easily detect them with TLC.
O H H C OH
N CH N CH3 O C 3 C O C O CH3CH2 O O CH3
Acetanilide Phenacetin Aspirin
Preparing the Plate:
Obtain two spotting capillaries and a TLC plate. Draw a light pencil line across the width of the plate, about 1 cm from the edge. Place 5 light “tic marks” on the line, approximately equally spaced.
Spotting Samples onto the Plate:
In separate test-tubes, dissolve tiny amounts of your aspirin and your recrystallized unknown in about 1 mL of acetone. Using one of the spotting capillaries, spot a tiny drop of the acetone solution of your aspirin on the left “tic-mark” - you may want to practice spotting on a paper towel or tissue, to make small spots. Allow it to dry, then check the plate under a short-wave UV light, to see if you have a visible spot. If not, spot your solution again on the same “tic-mark”. Do not spot any more times than needed - too much material may give results that are hard to interpret! Using a different capillary, spot the solution of your recrystallized unknown on the second to the leftmost “tic-mark”. After you have your samples spotted, take your plate to the standards in the counter, and spot acetanilide on the middle “tic-mark”, the phenacetin on the second to the rightmost “tic-mark”, and the aspirin on the rightmost “tic-mark”. Do not get the spotting capillaries contaminated! Check the spots under the UV light, to see if they are all visible.
Preparing the Developing Chamber:
To prepare a developing chamber, obtain a piece of 11 cm filter paper, and fold it about 1 inch from the edge, to obtain a flat edge. Place the filter paper, flat edge down, in your 400 mL beaker. Obtain 10 mL of the TLC solvent, methyl t-butyl ether, and pour it into the beaker, swirling the beaker to wet the filter paper with the solvent.
Developing the Plate:
Carefully place the TLC plate in the developing chamber, spotted side down, trying not to splash the solvent on the plate. The level of solvent must be below the pencil line. Cover the beaker with a watch 5 glass. The solvent will rise up through the stationary phase on the plate. When the solvent has risen to 1-2 cm from the top of the plate, remove the plate, and draw a light pencil line across the plate, at the level to which the solvent rose.
Identifying the Compounds:
Allow the solvent to evaporate (waving the plate in the air will speed this up), then look at the plate under the UV light. Circle all of the spots.
TLC Plate TLC Developing Chamber
Watch Glass Pencil line 1 cm from the end of the 400 mL Beaker plate. TLC plate Folded piece of 11 cm Filter paper
tic-marks Level of solvent below line of spots
You will identify the components of your unknown tablet by comparing the amounts the components traveled up the plate with the amounts the standards traveled. These amounts are reported as Rf (retention factor) values.
X Distance the spot traveled Rf = --- = ------Y Distance the solvent traveled
Measure the distance a spot moves from the center of the spot. 6
A developed plate may look like this:
From the locations of the spots, it would appear that the Your Aspirin (YA) contains Aspirin (A) and acetanilide (Ac). Perhaps Pencil line you didn’t do a good job of separation in the extraction. Your unknown (YU) only contains Ac. The spot for Ac in the standard looks larger than the spot for Ac in YU: this is probably due to different amounts of material being spotted on the plate initially. The Rf value for the A spot would be X/Y: measure X from the middle of the spot. Separate Rf values are calculated for Y each of the spots in YA. Since the spots for the materials in the samples and in the standards are different sizes and shapes, they may have slightly different Rf values.
X
YA YU Ac P A
Cleaning Up:
When you are finished with the experiment, pour the TLC solvent in the “Non-Halogenated Organic Solvent Waste” container. The filter paper may be thrown away in the trash can. Used spotting capillaries should be placed in the “Clean Broken Glass” container. Your acetone solutions you used to spot with may be flushed down the sink with lots of water.
Effect of Solvent on the Separation of the Panacetin Compounds
Prepare a second TLC plate, exactly like you did before. Your instructor will assign you one of the following solvents to use as a TLC solvent: toluene, acetone, ethyl acetate, or dichloromethane. Develop the plate in that solvent, and then circle the spots under the UV light.
Melting Ranges of Panacetin Products
Follow the melting point determination handout to record melting ranges.
Do the following melting ranges:
1. Aspirin 2. Your Unknown 3. Your Unknown + Phenacetin 4. Your Unknown + Acetanilide 7
Melting Range Determination
Melting range (sometimes just called melting point) is a fundamental physical property of a substance. A pure substance should have a melting range of 1-2 degrees. The melting range should also be close to the reported value in the chemical literature. Impure substances usually have wide melting ranges (over several degrees), and generally melt lower than the reported value.
There are several techniques and pieces of apparatus that can be used to take a melting range. We will be using the Meltemp apparatus. Although rather simple-looking, this apparatus costs over $800 to replace now, so handle it carefully!
The melting range of a substance is determined in the following manner.
1. Introduce a small amount (about 1-2 mm) of dry, finely powdered crystals into a capillary tube. Be sure that the crystals pack tightly. This can be accomplished by tapping the closed end of the capillary tube on the benchtop.
2. Place the capillary tube (closed end down) in the slot of the Meltemp®. Be careful not to break the tube in the apparatus. Notice that the Meltemp® can hold three tubes at once.
3. Heat the sample and record the melting range. This range starts when the sample just begins to liquify, and ends when the sample has totally liquefied. You will have to look in the eyepiece and watch the sample. When the sample just begins to liquefy, you will have to look up at the thermometer and record the temperature. You then look back down at the sample and watch it until it has totally liquefied, then look back up at the thermometer, and record this temperature.
The rate of heating is critical for obtaining a good melting range. If you heat the apparatus too fast, you may miss the end of the melting range, because the sample will have completely melted before you can look back down at it. Therefore, you should set a rate of heating of no more than about 5 per minute while your sample is melting. This is done by adjusting the voltage appropriately.
For example, let’s assume you have a sample that melts at 150. Looking at the table of heating curves, you can see that some voltage settings would not be appropriate. Voltages of less than 40 would never reach 150. Voltages of 80 or higher would have the temperature increasing so rapidly, you would miss the melting range. A voltage in the range of 50-60 would have a reasonable rate of temperature increase, and would probably work well. Notice that at 50 volts, it would take approximately 12 minutes to reach 150. (Note: on the blue apparatuses, the voltage knob only goes from 0-10. Divide the settings mentioned by 10 for the blue apparatuses.)
So what do you do if you don’t know what the melting range is supposed to be? One approach is to start with a reasonably low voltage (such as 50), then gradually increase the voltage to maintain a level of heating of about 5 per minute. The disadvantage of this approach is that it will probably take a long time if the melting point is >150. A second approach would be to take a melting range at a high voltage, such as 90, first. This melting range would be inaccurate, but would give you a rough idea of what range you are looking for. You would then go back and take a second melting range, using a more appropriate voltage setting. The disadvantage of this approach is having to use two samples, and the need to let the Meltemp® cool back down to about 20 below the melting point before you could use it again. Of course, if another Meltemp® is available, you could just use it. 8
Precautions:
1. The heating block of the Meltemp® gets very hot (surprise!). Therefore, do not touch it with your fingers or nose (yes, your nose can get close to it) when taking a melting range.
2. Check the temperature on the thermometer before you insert your capillary. If the temperature is higher than the melting point of your sample, it will melt immediately, and you will have to prepare another sample.
3. Don’t break off the capillary tubes in the Meltemp®. If you do, or if you find broken tube pieces in a Meltemp®, please notify your instructor so it can be cleaned out. Broken tubes reduce the efficiency of the apparatus. 9
Melting Range Exercises
1. Obtain three melting point capillaries. Put 1-2 mm of acetanilide into one capillary. Put 1-2 mm of dibenzalacetone into another capillary. Place a tiny amount of acetanilide and an equally tiny amount of dibenzalacetone in a small test-tube, and mix them together, then place 1-2 mm of this mixture into the third capillary. Place the three capillaries in a melting point apparatus, set it at a voltage of 50 (5 on the blue apparatuses), and record the melting ranges.
2. Turn off your apparatus, and allow it to cool until the thermometer reads 60 °C or less. Put 1-2 mm of acetanilide into one capillary. Place about 10 mm of acetanilide into another capillary. Place the tiniest amount of acetanilide possible (just enough to see) in the third capillary. Place the three capillaries in a melting point apparatus, set it at a voltage of 50 (5 on the blue apparatuses), and record the melting ranges.
3. Turn off your apparatus, and allow it to cool until the thermometer reads 60 °C or less. Put 1-2 mm of acetanilide into one capillary. Put 1-2 mm of isatin into another capillary. Place the two capillaries in a melting point apparatus, set it at a voltage of 80 (8 on the blue apparatuses), and record the melting ranges.
Literature Compound Melting Structure Point (°C) CH NH CH3 HC C C Acetanilide 114.3 HC CH O CH O
CH CH C CH CH Dibenzalacetone 113 HC C CH CH C CH
HC CH HC CH CH CH O
CH C HC C Isatin 201 C O HC C CH N H 10
Report Format
1. Title Page
a. Descriptive title with between 15-25 words. b. Course and section numbers. c. Dates the experiment was performed. d. Your name. e. Your partner’s name, if you had a partner.
2. Body of the report.
Exercise #1 Acetanilide melting range
Dibenzalacetone melting range
Acetanilide – Dibenzalacetone mixture melting range Exercise #2 1-2 mm sample melting range
10 mm sample melting range
Trace sample melting range
Exercise #3 Acetanilide melting range
Isatin melting range
3. Questions: You should read Operation 30 in the lab text to help you answer these questions.
a. In Exercise 1, why did the mixture of acetanilide and dibenzalacetone melt at a different range than either of the separate materials? Don’t just restate what is in the first paragraph of this handout: explain why.
b. Did the three samples in Exercise 2 melt over different ranges? If so, why? If not, why not?
c. Compare the 1-2 mm acetanilide samples’ melting ranges in Exercises 1, 2 and 3. How did they differ and why?
d. Assuming that the samples of acetanilide and dibenzalacetone are pure, why are the melting ranges you get different from the literature melting points? 11
Simple and Fractional Distillation Lab Modifications
Three pairs of you will form a group. One pair will do a simple distillation using 40 mL of a Methanol: Water mixture, and distilling out 30 mL. The second pair will do a fractional distillation with a packed column, using 40 mL of the Methanol: Water mixture and collecting 30 mL of distillate. The third pair will use a Vigreux’s all-glass column (http://www.rsc.org/chemistryworld/Issues/2008/April/VigreuxsColumn.asp ). You will record the temperature of the first drop of distillate, and then record the temperature every two milliliters of distillate until you have collected 30 mLs. Do not distill to dryness!
You will not collect samples for gas chromatography as stated in the procedure in the lab text. Simple and Fractional Distillation Report Form
1. Title Page
a. A descriptive title with between 15-25 words. b. Course and section numbers. c. Dates the experiment was performed. d. Your name.
2. Body of the report (start a new page)
a. Make a data table of Temperature versus Volume of Distillate for each type of distillation apparatus. Put all of the data for all three distillations in one table.
b. Plot a nice graph (on graph paper) of Temperature (on the vertical axis) versus Volume of Distillate (on the horizontal axis) for each type of distillation apparatus. Put all three sets of data on one graph. Do not “connect the dots”, but draw a nice curve through the data points for each apparatus. Make sure your graph is large enough to be read easily, shows the data points, is properly labeled, and distinguishes the data sets clearly. You can use Excel, but if you don’t know how to make Excel do all of this, then use graph paper.
3. Questions
a. From the data you collected, which distillation apparatus was the most efficient at separating the two liquids? Explain your reasoning using your data.
b. From your graph, what was the approximate composition of the Methanol: Water mixture? Explain your reasoning using your data.
c. The efficiency of a distillation can be improved by using a longer fractionating column. Roughly how long would you have to make the fractionating column to get close to an ideal separation of the two liquids? Explain your reasoning. There is no magic formula for determining this: estimate how much improvement the fractionating column made, and go from there. 12
Simple Distillation Apparatus
Water Out (to sink!)
Thermometer adapter and neoprene adapter Three-way Water In adapter
West Condensor Rubber Band Round-bottomed flask Vacuum adapter Thermowell
Transformer Plug into outlet 13
Fractional Distillation Apparatus
Water Out (to sink!)
Water In
Rubber Band
Thermowell
Transformer Plug into outlet 14
Properties of Common Functional Groups (14A & B, p. 115-122) & Who Else Has My Compound? (Minilab 14, p.473-4)
Remember, the goal of this lab is NOT to identify the compound exactly. The goals are to identify the functional group you have in your compound, and to find other people who have the same unknown as you have. Hence, you need to be careful when doing the various tests. You can repeat a test if need be. You will not get any more unknown, so be very careful with it!
Report format
1. Title Page
a. Descriptive title with between 10-25 words. b. Course and section numbers. c. Dates the experiment was performed. d. Your name.
2. Body of the report. a. Unknown number b. Boiling range c. Density Test Observation Conclusion d. Solubility in water e. Litmus test results, if done f. Results of 2,4-DNPH test g. Results of Chromic acid test h. Results of KMnO4 test i. Results of Beilstein’s test
3. Questions
a. What functional group was present in your unknown? Explain in detail how you determined this, using the data you obtained. b. Who else had the same unknown you did? How did you figure out who they were? c. Do exercises 3 and 6 on page 121. 15
Nitration of an Aromatic Compound and Recrystallization
Introduction:
In this experiment, you will do an aromatic nitration experiment. You will use one of the starting materials below. These only have one group on the benzene ring, so you have the possibility of three products: ortho-, meta-, and para-. You will use melting point as the primary method to determine the identity of your product.
Reaction Scheme:
G G
HNO3, H2SO4 ice bath NO2
Substituted benzene Nitrated product
The –NO2 off the side of the benzene ring means the NO2 group could be ortho, meta, or para to the G- group.
Possible starting materials:
Structure N O OCH Br 3 C C
Name Benzonitrile Bromobenzene Methyl Benzoate Melting Points of Possible Products ortho-nitro isomer 111 43 -13 meta-nitro isomer 118 56 78 para-nitro isomer 149 127 96 Most likely 129 75 112 dinitro isomer
Precautions:
Nitric acid is a strong acid and a powerful oxidizing agent. Sulfuric acid is a strong acid. Wear gloves. If you spill any of these, wipe them up with wet paper towels. If you spill these on you, wash the affected 16 areas with lots and lots of soap and water. Some of the organic starting materials and solvents are irritants, so wear gloves when handling these as well.
Reaction:
Place a small stir bar and 10 mL of concentrated sulfuric acid in a 125 mL Erlenmeyer flask, and cool the flask in an ice-water bath while stirring for about 10 minutes. Prepare a mixture of 3 mL of concentrated nitric acid and 3 mL of concentrated sulfuric acid in a large test-tube, and cool it in your ice-water bath. Measure out 40 mmoles of your starting material (record exactly how much you use): weigh out liquids into another test tube (how will you do this?) Add the starting material over a period of a minute to the Erlenmeyer flask. Keeping the Erlenmeyer flask in the ice-bath, add the cold mixture of nitric and sulfuric acids dropwise over about 10 minutes to the Erlenmeyer flask, while stirring the flask. After you have added the nitric and sulfuric acids, continue to stir the flask in the ice bath over the next 30 minutes.
Product Isolation and Purification:
Pour the reaction mixture, with stirring, into about 20 grams of ice in a small beaker. The product should precipitate as you stir the mixture. Collect the product by suction filtration, and wash it thoroughly with three small portions of ice-cold water. Remember to release the suction, add the wash solution and gently stir the wash liquid with the solid, and reapply the suction.
Recrystallize the product from methanol (benzonitrile or methyl benzoate products) or 95% ethanol (bromobenzene product). Start with about 10 mL of solvent, and only use more if the solid does not dissolve when boiling. Once it has all dissolved in the boiling solvent, allow the flask to cool to room temperature, then cool it further in an ice-bath. Collect the product by suction filtration, and wash the product with a few mL of ice-cold solvent. Allow air to pull through the crystals for a few minutes. Transfer a small amount of crystals to a watch glass, and then recrystallize the rest of your product again. Transfer the twice-recrystallized product to another watch glass. Allow the products to dry for a few days further. When the crystals are thoroughly dry, measure their mass and melting point range. Place the twice-recrystallized crystals in a vial labeled as follows:
Name of product Mass Your Name(s)
TLC Analysis:
You will analyze your product for purity by TLC. Dissolve a tiny amount of your starting material and your recrystallized products in acetone in separate test-tubes. Spot these on a TLC plate, and look at the spots under the UV light to make sure they are visible. Develop the plate using dichloromethane as the TLC solvent. When the solvent is 1-2 cm from the top, remove the plate, wave the plate around in the air to evaporate the solvent, and look at it under the UV light, circling the spots. Include your plate with your report. 17
Report Format
1. Title Page
a. Descriptive title with between 10-25 words. b. Course and section numbers. c. Dates the experiment was performed. d. Your name. e. Tape your TLC plate to the bottom of this page. Clearly label each column of spots so I know what they are.
2. Body of the report.
a. A balanced chemical equation for the reaction you did. b. Important observations. c. Melting point ranges of the two sets of crystals. d. Mass of the twice-recrystallized product. e. Calculations of theoretical and percent yields, using the exact amounts of starting materials you used. Calculate the moles of each starting material, and determine the limiting reagent. Show your work. f. Calculate Rf values for each of the spots on the TLC plate.
3. Questions
a. Which nitrated isomer of your starting material did you isolate? Explain your reasoning, using the data you acquired, as well as the directing effects of the group on the benzene ring. b. Why did this isomer form in preference to the other isomers? Draw arrow-pushing mechanisms as a major part of your explanation. c. What is the most likely dinitroproduct from your starting material? Explain why this isomer should be the most likely. d. For each of the following compounds, draw the structure of the major nitration product: just substitute one NO2 group. Explain your reasoning: you don’t have to draw mechanisms for this question – you just need to know and apply directing-group abilities.
NH2 O S O H Cl Cl N C Cl O 18
Reduction of Nitro Compounds with Copper (II) Acetate and Sodium Borohydride
Recently, an article was published by Biga and Gibbs1 that claimed aromatic nitro compounds could be reduced with copper (II) acetate and sodium borohydride in ethanol. When I attempted to repeat the experiment, I didn’t have as much success as was claimed in the article. When I attempted to contact the principle author, Don Gibbs, I discovered that he has passed away early in 2008. I could not locate the other author. Biga and Gibbs referenced an earlier article by Cowan2, but the procedure in Cowan was incomplete. Therefore, we are going to attempt to reproduce the success of Biga and Gibbs by modifying the reaction conditions. We will also monitor the course of the reaction by TLC.
General Reaction:
OCH3 OCH3
NaBH4, NO2 NH2 Cu(OAc)2 95% ethanol ortho-nitroanisole
CH3 CH3
NaBH4,
Cu(OAc)2 95% ethanol NO2 NH2 meta-nitrotoluene
Procedure:
Prepare a TLC plate by drawing a pencil line across the width of the plate, about 1 cm from the end. Make 6 marks on the line approximately equally spaced. On one of the marks, spot the TLC standard of your nitro compound. On another mark, spot the TLC standard of your amine.
To a 125 mL Erlenmeyer flask, add a stir bar, 1.0 g (record what you actually weigh out) of your assigned nitro compound, 0.5g of copper (II) acetate monohydrate (Cu(OAc)2•H2O), and 30 mL of 95% ethanol. Allow the mixture to stir for 5 minutes. Using a three-fingered clamp, position a thermometer so that you can record the temperature of the reaction, without the thermometer being hit by the stir bar. Weigh out your assigned amount of sodium borohydride, and add one-quarter of it every 20 minutes. Ten minutes after each addition of sodium borohydride, spot your reaction mixture on a different mark on your TLC plate – if you clean out the capillary, you can use the same spotting capillary each time. Look at the plate under the UV light each time, to see if you have a dark spot. After you have spotted the last spot, develop your TLC plate using dichloromethane (CH2Cl2, “methylene chloride”) as the solvent. After the plate has developed, look at the plate under a UV light, and circle all of the spots. Get the TLC data of the other groups who used the same nitrocompound you did.
Twenty minutes after the last addition of sodium borohydride, add the following solutions to your reaction in this order: 20 mL of 5% NaHCO3, 30 mL of water, and 30 mL of ethyl ether. Stop the stirrer, and allow the black precipitate to settle. Filter the solution by suction filtration, and transfer the filtrate 19
to your separatory funnel. Rinse the reaction flask with 30 mL of ether, and filter it by suction as well, then transfer the filtrate into the separatory funnel. Place the solid in the beaker in the hood.
Returning to the separatory funnel, gently shake and vent a few times, allow the layers to separate, and drain off the lower aqueous layer. Wash the ether layer successively with 20 mL of water and with 20 mL of saturated sodium chloride solution. Pour the top ether layer into dry 125 mL Erlenmeyer flask, add two spatulas-full of anhydrous sodium sulfate, then swirl the flask occasionally for 5 minutes – this is called “drying the ether layer”. Filter the ether solution through a plug of cotton in dry powder funnel into a pre-weighed and dry 100 mL round-bottomed flask. Remove the ether layer on the rotary evaporator, and reweigh the flask.
With the help of an instructor, take an IR spectrum of your product. The N-H stretches of an NH2 often shows up as two peaks about 3300 cm-1. An aromatic nitro group’s N-O stretches often show up as two peaks about 1520 and 1350 cm-1.
Turn in your product in a screw-capped vial, labeled with the product name, mass, and your names.
References:
1. An Efficient Reduction of ortho-Nitroanisole. Ryan Biga and Don E. Gibbs, Missouri Journal of Undergraduate Chemical Research 2007/2008, 9, 28-30. 2+ - 2. Cu /BH4 Reduction System: Synthetic Utility and Mode of Action. Cowan, J. A. Tetrahedron Letters 1986, 27(10), 1205-8.
Group Number Nitro Compound Amount of Sodium Borohydride 1 ortho-nitroanisole 2.0 g 2 ortho-nitroanisole 3.0 g 3 ortho-nitroanisole 4.0 g 4 meta-nitrotoluene 2.0 g 5 meta-nitrotoluene 3.0 g 6 meta-nitrotoluene 4.0 g 20
Report Format for Reduction of Nitro Compounds
A. Title Page:
1. Descriptive title containing 15-25 words. 2. Your name. 3. Course and section numbers. 4. Dates the experiment was performed. 5. Tape your TLC plate to the bottom of the page. Clearly label each column so I know what it is.
B. Body of the Report (Start a new page)
1. Chemical equation, using your real starting material: you don’t have to balance it. 2. Observations (things seen, smelled, etc.), with possible explanations. 3. Weight of your product. 4. Calculations of moles of all reactants from the grams or milliliters of each reactant you actually started with: show your work. Copper acetate is a catalyst. 5. Calculations of theoretical yield and percent yield. Show your work. 6. Calculate the Rf values for each of the spots on your TLC plate. Show your work. 7. Attach your IR spectrum. Label the peaks for any amines or nitro groups that appear in your spectrum.
C. Questions
1. Did your reaction completely reduce the nitro group to an amine? Explain in detail how you determined this, using data you acquired. 2. How much sodium borohydride was required to completely reduce the nitro-group in your compound? What is the mole ratio of nitro-compound to sodium borohydride required for complete reduction? 3. Why did we add the sodium borohydride in portions at twenty minute intervals? Why not all at once?