SEKOLAH MENENGAH KEBANGSAAN RAJA PEREMPUAN , IPOH. SCHEME OF WORK CHEMISTRTY FORM 5 2010
THEME : INTERACTION BETWEEN CHEMICALS LEARNING AREA : RATE OF REACTION
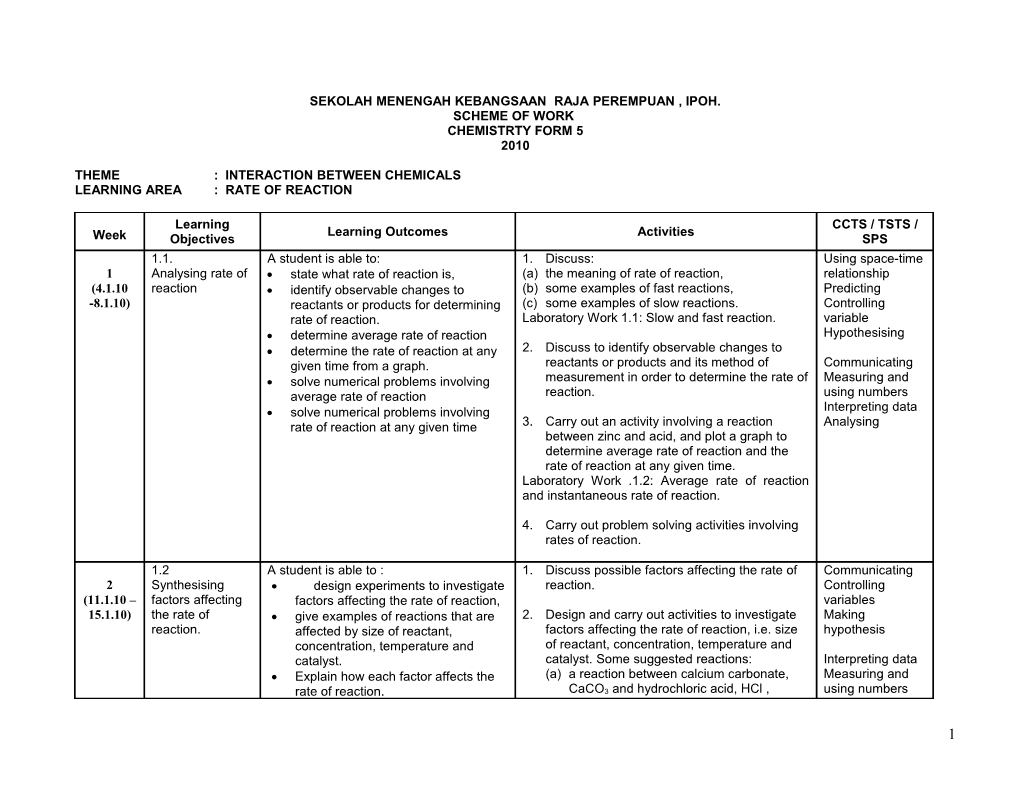
Learning CCTS / TSTS / Learning Outcomes Activities Week Objectives SPS 1.1. A student is able to: 1. Discuss: Using space-time 1 Analysing rate of state what rate of reaction is, (a) the meaning of rate of reaction, relationship (4.1.10 reaction identify observable changes to (b) some examples of fast reactions, Predicting -8.1.10) reactants or products for determining (c) some examples of slow reactions. Controlling rate of reaction. Laboratory Work 1.1: Slow and fast reaction. variable determine average rate of reaction Hypothesising determine the rate of reaction at any 2. Discuss to identify observable changes to given time from a graph. reactants or products and its method of Communicating solve numerical problems involving measurement in order to determine the rate of Measuring and average rate of reaction reaction. using numbers solve numerical problems involving Interpreting data rate of reaction at any given time 3. Carry out an activity involving a reaction Analysing between zinc and acid, and plot a graph to determine average rate of reaction and the rate of reaction at any given time. Laboratory Work .1.2: Average rate of reaction and instantaneous rate of reaction.
4. Carry out problem solving activities involving rates of reaction.
1.2 A student is able to : 1. Discuss possible factors affecting the rate of Communicating 2 Synthesising design experiments to investigate reaction. Controlling (11.1.10 – factors affecting factors affecting the rate of reaction, variables 15.1.10) the rate of give examples of reactions that are 2. Design and carry out activities to investigate Making reaction. affected by size of reactant, factors affecting the rate of reaction, i.e. size hypothesis concentration, temperature and of reactant, concentration, temperature and catalyst. catalyst. Some suggested reactions: Interpreting data Explain how each factor affects the (a) a reaction between calcium carbonate, Measuring and rate of reaction. CaCO3 and hydrochloric acid, HCl , using numbers
1 Learning CCTS / TSTS / Learning Outcomes Activities Week Objectives SPS Describe how factors affecting the (b) a reaction between sodium thiosulphate, rate of reaction are applied in daily Na2S2O3 and sulphuric acid, H2SO4, life and in industrial processes. (c) decomposition of hydrogen peroxide,H2O2 Communicating Solve problems involving factors in the presence of a catalyst. Defining affecting rate of reaction. Experiment 1.1: Effect of surface area on the rate operationally of reaction. Analysing Experiment 1.2: Effect of concentration on the rate of reaction. Synthesising Experiment 1.3: Effect of temperature on the rate of reaction. Experiment 1.4: Effect of catalyst on the rate of reaction. Experiment 1.5: Effect of the amount of catalyst on the rate of reaction.
3. View computer simulations to investigate how the movement and collision of particles in a reaction are affected by temperature, size of reactant, pressure, concentration and catalyst.
4. Collect and interpret data to explain factors affected the rate of reaction in the following : (a) combustion of charcoal, (b) storing food in a refrigerator, (c) cooking food in a pressure cooker, (d) industrial production of ammonia, sulphuric acid and nitric acid.
5. Solve problems involving rate of reaction.
3 1.3 A student is able to: 1. Carry out simulations on: (18.1.10- Synthesising relate reaction with energy produced (a) movement and collision of particles in Generating ideas 22.1.10) ideas on collision by movement and effective collision of chemical reactions, Relating theory particles. (b) movement and describe activation (c) collision of particles in reaction affected Grouping and energy, by temperature, size of reactant, pressure interpreting data sketch and describe concentration and catalyst. Conceptualising energy profile diagram, Visualising
2 Learning CCTS / TSTS / Learning Outcomes Activities Week Objectives SPS relate the frequency of 2. Collect, interpret data and discuss the effective collision with the rate of following: Synthesising reaction, (a) collision relate the frequency of effective (b) effective collision, collisions with factors influencing the (c) activation energy, rate of reaction, (d) collision frequency, describe how a certain (e) effective collision frequency, factor affects the collision of particles in (f) energy profile diagram. a reaction. 3. Discuss to conceptualise collision theory
1.4 A student is able to: 1. Carry out some daily activities related to Relating Practising Apply knowledge on factors affecting factors affecting the rate of reaction. Evaluating scientific the rate of reaction in everyday 2. Collect and interpret data on scientists’ Problem solving knowledge to activities. contribution in enhancing the quality of life enhance quality Adopt problem solving approaches and 3. Carry out problem solving activities involving of life make rational decisions based on rate of reaction in the field of science and research technology through experiment and research.
3 THEME : INTERACTION BETWEEN CHEMICALS LEARNING AREA : 2. CARBON COMPOUNDS
Learning TSTS/ CCTS/ Learning Outcomes Activities Week Objectives SPS 2.1 A student is able to: 1. Collect and interpret data on: Predicting 5 Understanding State what carbon compound is, (a) the meaning of carbon compound, Communicating (25.1.10- Carbon State the carbon compounds can be (b) the meaning of organic compound with Characteristing 29.1.10) Compounds classified into two groups, i.e. respect to its sources, content and Relating organic and inorganic, combustion products, State what organic compound is, (c) the meaning of hydrocarbon, inclusive of Gives examples of organic and saturated and unsaturated hydrocarbons, inorganic carbon compounds, (d) sources of hydrocarbon, State what a hydrocarbon is, (e) examples of organic and inorganic List the sources of hydrocarbon, compounds. Identify the combustion products of organic carbon compounds. 2. Carry out an activity to identify the products of Experimenting the combustion of organic compounds, i.e. Communicating carbon dioxide and water. Making Laboratory work 2.1: Combustion products of conclusion organic compounds. Use and handle science apparatus and laboratory substances correctly Relating Making inferences Making inferences Generating ideas
4 Learning TSTS/ CCTS/ Learning Outcomes Activities Week Objectives SPS 2.2 A student is able to : 1. Collect and interpret data on : Making Analysing state what alkane is, (a) the meaning of alkane, generalization alkanes state what structural formula is, (b) the meaning of structural formula, Sequencing deduce the molecular formulae of 2. Carry out an activity to construct molecular the first ten alkanes models and draw structural formulae of the first Relating Draw the structural formulae for the ten straight-chain alkanes. Comparing and first ten straight-chain alkanes, 3. Construct a table showing names, molecular contrasting Deduce the general formula of formulae, structural formulae and physical alkanes properties i.e. melting and boiling points, Predicting Name the first ten alkanes, density, physical state at room temperature, solubility in water and electrical conductivity of Relate changes in physical the first ten straight-chain alkanes. properties with increase in the 4. Discuss : number of carbon atoms in alkanes (a) the relationship between changes in molecules physical properties with increase in the Explain the effect of the increase in number of carbon atoms in alkanes number of carbon atoms in alkanes molecules. Classifying molecules on the molecules boiling (b) the effect the boiling points of alkanes due points, to increase in the number of carbon atoms Describe complete and incomplete in alkanes molecules combustion of alkanes, 5. Collect and interpret data on chemical Describe the substitution reaction of properties of alkanes, i.e. combustion, alkanes, substitution reaction with halogen. (using Write chemical equations for methane as example) combustion and substitution reaction 6. Discuss the effect on: of methane, (a) the combustion of alkanes Describe how methane affects (b) substitution reaction of alkanes due to Compare and everyday life. increase in the number of carbon atoms in contrasting alkanes molecules. 7. Write chemical equation for combustion and substitution reaction of methane 8. Group discussion and presentation on Predicting decomposition of organic matter produces methane and how this may cause fire in land Generating ideas fills and peat swamps.
5 Learning TSTS/ CCTS/ Learning Outcomes Activities Week Objectives SPS 6 2.3 A student is able to: 1. Collect and interpret data on the meaning of Interpreting data (1.2.10- Analysing state what alkene is, alkene 5.2.10) alkenes deduce the molecular formulae of the 2. Carry out an activity to construct molecular Experimenting first nine alkenes, models and draw structure formulae of the first deduce the general formula of straight-chain alkenes with one double bond . Comparing and alkenes, 3. Construct a table showing names, molecular contrasting name the first nine alkenes, formulae , structural formulae and physical draw the structural formulae for the properties of the first nine straight-chain Analysing first nine straight –chain alkenes, alkenes. relate changes in physical properties 4. Collect and interpret data on: Detecting bias with increase in the number of (a) physical properties of alkenes, i.e melting carbon atoms in alkenes molecules, and boiling points, density, physical state Making at room temperature , solubility in water conclusion explain the effects on boiling points and electrical conductivity, of alkenes due to increase in the (b) chemical properties of alkenes i.e Generalising number of carbon atoms in alkenes combustion , addition reaction and molecules, polimerisation . Evaluating describe chemicals properties of 5. Discusss: alkenes, (a) the relationship between changes of physical compare and contrast alkanes with properties with increase in the number of alkenes, carbon atoms in alkenes molecules, relate the reactivities of alkanes and (b) how the increase in the number of carbon alkenes to their chemicals bonds. atom in alkenes , affect their boiling points Generalise the characteristics of (c) the combustion of alkenes homologous series based on alkanes (d) the addition reaction of alkenes and alkenes (e) the polimerisation of alkenes
6. Write chemical equations for the combustion, addition and polimerisation reaction of alkenes. 7. Carry out activities to compare properties of alkanes and alkenes having the same number of carbon atom such as hexane , C6H14, and hexene, , C6H12, with respect to : (a) sootiness of flame, (b) reactions with bromine Br2 , (c) reaction with acidified potassium manganate(VII), KMnO4 . 8. Compare qualitatively the sootiness of flame during combustion of an alkane with the
6 Learning TSTS/ CCTS/ Learning Outcomes Activities Week Objectives SPS corresponding alkene. Laboratory Work 2.2: Properties of alkanes and alkenes. 9. Discuss to generalise the characteristics of homologous series in terms of having the same general formula, can be made by similar methods, steady changes in physical properties, and similar chemical properties
2.4 A student is able to: 1. Construct all possible models and draw Generating ideas Synthesising Construct various structural formulae structural formulae for a particular alkane and Conceptualising ideas on of a particular alkane and alkene. alkene. Comparing and isomerism Explain what isomerism is 2. Construct a table showing names and formulae contrasting Use IUPAC nomenclature to name of alkyl groups. isomers 3. Discuss isomerism. Visualising 4. Discuss the existence of isomers. Sequencing 5. Draw structural formulae of alkane and alkene Systematic isomers and name them. Conceptualising 6. Examine isomerism through models or computer simulations.
A student is able to: 1. Carry out an activity to derive general formula generating ideas 7 2.5 state the general formula of alcohols, of alcohols and identify the functional group. observing (22.2.10- Analysing identify the functional group of 2. Construct a table of names and molecular classifying 25.2.10) alcohols alcohols, formulae for the first four alcohols. list the names and molecular formulae 3. Carry out an activity to draw various possible for the first four alcohols, structural formulae of the first four alcohols and draw structural formulae for isomers name them. making 4. Collect and interpret data on the industrial inferences of propanol (C3H7OH) and butanol production of ethanol. (C4H9OH), name isomers of propanol and butanol 5. Carry out an activity on the preparation of using IUPAC nomenclature, ethanol in the laboratory through fermentation describe the industrial production of and distillation. interpreting data ethanol. Laboratory Work 2.3: Preparation of ethanol by fermentation. describe the preparation of ethanol in the laboratory, 6. Collect and interpret data on the physical
7 Learning TSTS/ CCTS/ Learning Outcomes Activities Week Objectives SPS state the physical properties of properties of ethanol (C2H5OH), i.e. colour, ethanol, odour, boiling point, physical state at room predict the chemical properties for temperature, volatility and solubility. other members of alcohols, explain with examples the uses of 7. Carry out activities to investigate the chemical alcohol in everyday life, properties of ethanol in terms of : explain the effects of the misuse and (a) combustion, relating abuse of alcohols. (b) oxidation experimenting (c) dehydration Laboratory Work 2.4: Chemical properties of generating ideas ethanol. classifying attributing 8. Write chemical equations for the above reactions involving ethanol, propanol and butanol. 9. Carry out an activity to predict the chemical Experimenting properties for other members of alcohols. 10. Collect and interpret data on: Predicting (a) uses of alcohol in everyday life, (b) effects of alcohol misuse and abuse. Interpreting data
Relating
2.6 A student is able to : 1. Carry out activity to derive the general formula Generating ideas 8 Analysing state the general formula of carboxylic of carboxylic acids and identify the functional making (1.3.10 carboxylic acids acids, group. hypotheses 5.3.10) identify the functional group of 2. Construct a table with names and molecular carboxylic acids, formulae of the first four members of carboxylic list the names and molecular formulae acid, and draw their structural formulae Relating of the first four members of carboxylic 3. Collect and interpret data on the preparation of attributing acid, ethanoic acid (CH3COOH) in the laboratory classifying draw structural formulae of the first 4. Collect and interpret data on the physical four members of carboxylic acid and properties of ethanoic acid, i.e. colour, odour, name them using the IUPAC boiling point, physical state at room nomenclature, temperature and solubility in water describe the preparation of ethanoic 5. Carry out activities to investigate the chemical acid in the laboratory, properties of ethanoic acid through its reactions state the physical properties of with : (a) base,
8 Learning TSTS/ CCTS/ Learning Outcomes Activities Week Objectives SPS carboxylic acids, (b) metallic carbonate, Making inference state the chemical reactions of (c) metal, predicting ethanoic acids with other chemicals, (d) alcohol. visualising predict the chemical properties for Laboratory Work 2.5: Chemical properties of other members of carboxylic acid, ethanoic acid Experimenting explain with example the uses of 6. Carry out an activity to write chemical carboxylic acids in everyday life equations for the above reactions involving propanoic acid (C2H5COOH) and butanoic acid (C3H7COOH) 7. Carry out an activity to predict the chemical properties of other members of carboxylic acids 8. Collect and interpret data on the uses of Predicting carboxylic acids in everyday life.
9 REVISION (9.3.10- 12.3.10) (9.3.10-12.3.10 USBF 1
MID TERM BREAK (13.3.2010 – 21.3.2010)
2.7. A student is able to: 1. Carry out an activity to derive the general Generating idea 10 Analysing Esters state the general formula of esters, formula of esters and identify the functional interpreting data (22.3.10- identify the functional group of esters, group. relating 26.3.10) list the names and molecular formulae 2. Construct a table of molecular formulae and of simple esters, names of esters. Draw structural formulae of simple 3. Carry out an activity to prepare ethyl ethanoate esters and name them using the (CH3COOC2H5) in the laboratory. IUPAC nomenclature, 4. Carry out an activity to investigate the physical Describe the preparation of ester in the properties of ethyl ethanoate, i.e. the odour and laboratory, solubility. State the physical properties of ethyl ethanoate, Laboratory Work 2.6; Esters – Laboratory
9 Learning TSTS/ CCTS/ Learning Outcomes Activities Week Objectives SPS Predict the ester produced from preparation and physical properties. the esterification reaction, 5. Discuss to predict the esters produced from the Experimenting Write equations for the esterification esterification between various carboxylic acids attributing reactions, and alcohols. state the natural sources of ester, 6. Write equations for esterification reactions. Predicting state the uses of ester in everyday life. 7. Collect and interpret data on: Making inference (a) natural sources of ester visualising (b) uses of ester in everyday life. 8. Carry out a project to extract esters from plants.
11 2.8 A student is able to: 1. Group activity: Grouping and (29.3.10- Evaluating fats State what oils are Collect and interpret data on: classifying 2.4.10) State what fats are (a) what oils and fats are Comparing and State the importance of oils and fats (b) why our body needs oils and fats contrasting for body processes (c) sources and the uses of oils and fats Evaluating State the sources of oils and fats (d) the difference between oils and fats at List the uses of oils and fats room temperature in terms of physical State the differences between oils state and fats (e) structural formulae for fat molecules of certain fatty acids Identify structural formulae for fat 2. Group activity: molecules of certain fatty acids Collect and interpret data on: State what saturated fats are (a) what saturated and unsaturated fats are Grouping and State what unsaturated fats are (b) sources and compositions of saturated and classifying Compare and contrast between unsaturated fats Comparing and saturated and unsaturated fats (c) the differences between saturated and contrasting Describe the effects of eating food unsaturated fats Generalising high in fats on health (d) the need to convert unsaturated to Evaluating Describe the industrial extraction of saturated fats palm oil (e) effects of fats on health Justify the use of palm oil in the food 3. Discuss the production of margarine by Visualising production hydrogenation, 4. Visit a palm oil factory, margarine manufacturing plant or palm oil research institute. 5. Panel speakers: Discuss:
10 Learning TSTS/ CCTS/ Learning Outcomes Activities Week Objectives SPS (a) the advantages of palm oil as compared to other vegetable oils (b) research on oil palm in Malaysia Justifying (c) the importance of palm oil industry to the development of the country
12 2.9 A student is able to : 1. Collect and interpret data on : (5.4.10- Analysing natural List examples of natural polymers and (a) natural polymer i.e natural rubber, starch Prioritising 9.4.10) rubber their monomers, and protein and their respective monomers Draw the structural formula of natural (b) properties of natural rubber in terms of rubber, elasticity, oxidation and the effects of heat State the properties of natural rubber, and solvents. Synthesising State the uses of natural rubber (c) Uses of natural rubber, Describe the coagulation process of (d) Structural formula of natural rubber. Attributing latex 2. Carry out an activity to investigate the Attributing Describe the method used to prevent coagulation of latex and methods to prevent latex from coagulating, coagulation. Laboratory Work 2.7: Coagulation of latex Defining Describe the vulcanization of rubber, 3. Carry out activities to produce latex products operationally Describe how the presence of sulphur such as gloves and balloons. atoms changes the properties of 4. Carry out an activity to produce vulcanised vulcanised rubber, rubber. Defining Compare and contrast the properties Laboratory Work 2.8: Vulcanised rubber operationally of vulcanised and unvulcanised 5. Investigate the process of rubber vulcanisation natural rubber. using computer simulation. 6. Discuss : (a) how the presence of sulphur atom in vulcanized rubber changes the properties Defining of vulcanized rubber. operationally (b) Research on natural rubber in Malaysia 7. Carry out an activity to compare the elasticity of vulcanised and unvulcanised natural rubber. Experiment 2.1: Elasticity of vulcanised and unvulcanized rubber. Laboratory Work 2.9: Latex product. 8. Visit a rubber plantation, a latex processing factory, a rubber product manufacturing plant Defining or a rubber research institute. operationally
11 Learning TSTS/ CCTS/ Learning Outcomes Activities Week Objectives SPS 2.10 A student is able to : 1. Construct a table naming each member of the Comparing and Creating describe the systematic approach in homologous series according to the increasing contrasting awareness of naming members of homologous number of carbon atoms. Attributes order in series. 2. Discuss the order in the physical and chemical Predicting homologous describe the order in the physical and properties and compounds in homologous Inferring series chemical properties in homologous series. Sequencing series. Classifying 3. View computer simulations about the subtopic
2.11 A student is able to : 1. Collect and interpret data on the existence of a Expressing describe the existence of various variety of organic materials in consumer gratefulness for organic materials and their uses in products. the variety of everyday life 2. Attend activities (talks, forum, exhibition) Generating ideas organic materials practice good nutrition for health related to good nutrition for health. Interpreting data in nature relate the contribution of palm oil and 3. Conduct a forum related to the contribution of natural rubber to the economic palm oil and natural rubber industries with the development of the country country’s economy.
THEME : INTERACTION BETWEEN CHEMICALS LEARNING AREA : 3. OXIDATION AND REDUCTION
12 Learning CCTS / TSTS / Learning Outcomes Activities Week Objectives SPS 13 3.1 A student is able to: 1. Collect and interpret data on oxidation, Defining (12.4.10- Analysing redox state what oxidation is, reduction, redox reaction, oxidising agent and operationally 16.4.10) reactions state what reduction is, reducing agent based on Classifying explain what redox reaction is, (a) loss or gain of oxygen 14 state what oxidising agent is, (b) loss or gain of hydrogen Interpreting data (20.4.10- state what reducing agent is, (c) transfer of electron Communicating 23.4.10) calculate the oxidation number of an (d) change in oxidation number data element in a compound, Laboratory Work 3.1: Redox reaction as loss or gain of oxygen. Measuring and relate the oxidation number of an Acttivity 3.1: Oxidation and reduction as loss or using number element to the name of its compound gain of oxygen or hydrogen. Relating using the IUPAC nomenclature, Activity 3.2: Oxidation and reduction as transfer of Evaluation explain with examples oxidation and electrons. reduction processes in terms of the change in oxidation number, 2. Calculate the oxidation number of an element explain with examples oxidation and in a compound. reduction processes in terms of electron Activity 3.3: Oxidation number. transfer, explain with examples oxidation and 3. Carry out an activity to identify the oxidation reducing agents in redox reactions, number of an element in a compound and write oxidation and reducing half- name the compound using the IUPAC equations and ionic equations. nomenclature. Activity 3.4: Naming of compounds.
4. Carry out an activity to identify oxidation and reduction processes in chemical equations: (a) using oxidation number Observing (b) in terms of electron transfer Making inferences 5. Carry out activities to investigate oxidation Experimenting and reduction in the followinng reactions: Making a. combustion of metals or chlorine, conclusion b. heating of metallic oxide with carbon, Predicting c. change of Fe2+ions to Fe3+ and Fe3+ to Controlling Fe2+ variables d. displacement of halogen from its halide Communicating solution, e. transfer of electrons at a distance (a Grouping and
13 Learning CCTS / TSTS / Learning Outcomes Activities Week Objectives SPS variety of solutions to be used). classifying Laboratory Work 3.2: Change of iron(II) ions to iron(III) ions and vice versa. Synthesising Laboratory Work 3.3: Displacement of metals. Grouping and Laboratory Work 3.4: Displacement of halogens. classifying Laboratory Work 3.5: Transfer of electrons at a distance. Activity 3.5: Half equations and ionic equations. Activity 3.6: Examples of oxidising and reducing agents.
15 3.2 A student is able to: 1. Collect and integrate data on : Making (26.4.10- Analysing rusting State the conditions for the rusting of a. Conditions for the rusting of iron, hypothesis 30.4.10) as a redox iron, b. The meaning of corrosion of metal, Analysing reaction State what corrosion of metal is c. The process of rusting in terms of Relating Describe the process of rusting in oxidation and reduction. Making terms of oxidation and reduction, inferences Generate ideas on the use of other 2. Discuss the redox reactions in corrosion of Predicting metals to control rusting, metals including rusting. Making Explain with examples on the use of a conclusions more electropositive metal to control 3. Discuss on the use of other metals to control metal corrosion, rusting. Explain with examples on the use of a less electropositive metal to control 4. Carry out an activity to investigate the effect Making metal corrosion. on iron nails when it is in contact with other hypothesis metals. Controlling Experiment 3.1: Effects of other metals on rusting. variables 5. Collect and interpret data on methods to Experimenting control metal corrosion using a more electropositive metal or a less electropositive Communicating metal. Observing Activity 3.7: Rusting and controls of rusting. Making Activity 3.8: Control of rsuting. inferences Predicting Interpreting data
14 Learning CCTS / TSTS / Learning Outcomes Activities Week Objectives SPS 16 3.3 A student is able to: 1. Carry out an activity to investigate the Observing (3.5.10- Understanding compare the differences in the vigour reactivity of some metals with oxygen. Inferring 7.5.10) the reactivity of the reactions of some metals with Laboratory Work 3.6: Reactivity of metals with series of metals oxygen. oxygen. Sequencing and its deduce the reactivity series of metals. Analyzing application determine the position of carbon and 2. Arrange metals in terms of their reactivity with Comparing and hydrogen in the reactivity series of oxygen. contrasting metals. state what the reactivity series of 3. Carry out activity to determine the position of metals are. carbon and hydrogen in the reactivity series of describe the extraction of iron and tin metals. from their ores. Laboratory Work 3.7: The position of carbon in the explain the use of carbon as the main reactivity series of metals. reducing agent in metal extraction. Laboratory Work 3.8: The position of hydrogen in the reactivity series of metals. use the reactivity series of metals to predict possible reactions involving 4. Discuss to predict the position of other metals metals. in the reactivity series.
5. Collect and interpret data on the extraction of iron and tin.
6. Visit metal extraction factories or view a video on the extraction of metals. Activity 3.9: Extraction of metal. Activity3.10: Extraction of iron and tin. 7. Discuss the use of the reactivity series of metals to predict possible reactions involving metals. 8.
17 3.4 A student is able to: 1. Carry out an activity to investigate oxidation Attributing (10.5.10- Analysing redox explain with examples the oxidation and reduction reactions in electrolytic and Comparing and 14.5.10) reactions in and reduction reactions at the chemical cells. contrasting electrolytic and electrodes of various chemical cells, Activity 3.12: Electrolytic and chemical cells. chemical cells explain with examples the oxidation Conceptualizing and reduction reactions at the 2. Using computer simulation, study and discuss electrode of various electrolytic cells, redox reactions in various types of cells.
15 Learning CCTS / TSTS / Learning Outcomes Activities Week Objectives SPS state the differences between electrolytic and chemical cells in terms 3. Discuss the differences between electrolytic of basic structure, energy conversion and chemical cells in terms of: and the transfer of electrons at the (b) basic structure, energy conversion and electrodes, the transfer of electrons at the electrodes, compare and contrast electrolytic and (c) oxidation and reduction processes. chemical cells with reference to the Laboratory Work 3.9: Oxidation and reduction in oxidation and reduction processes. electrolytic cells. Laboratory Work 3.10: Oxidation and reduction in chemical cells.
17 3.5 A student is able to: (17.5.10- Appreciating the Describes the various applications of 21.5.10) ability of the change of oxidation number in elements to substances, change their Describe the existence of various oxidation types of ores in our country numbers. Describe efforts to prevent corrosion of metals, Describe the contribution of metal extraction industry to the economy of our country Appreciate chemical cell as a source of renewable energy
18-20 (13.5.10- Revision 27.5.10) MID YEAR EXAMINATION
MID YEAR BREAK
(31.5.2010– 13.6.2010)
16 THEME : INTERACTION BETWEEN CHEMICALS LEARNING AREA : 4 THERMOCHEMISTRY
Learning CCTS / TSTS / Learning Outcomes Activities Week Objectives SPS 4.1 A student is able to: 1. Discuss the meaning of exothermic and Making 21 Evaluating State what exothermic reaction is endothermic reactions. hypothesis (14.6.10- energy changes State what endothermic reaction is Activity 4.1: Exothermic and endothermic reaction Controlling in chemical variables 18.6.10) Identify exothermic reactions reaction 2. Carry out activities to study exothermic and Experimenting Identify endothermic reactions endothermic reactions in the: Communicating Give examples of exothermic (a) Reaction between sodium hydrogen Observing reactions Interpreting data carbonate, NaHCO3, and an acid, Give examples of endothermic (b) Reaction between sodium hydroxide Grouping and reactions NaOH, and hydrochloric acid, HCl classifying Making Construct energy level diagrams for (c) Dissolving of sodium hydroxide in water conclusion exothermic reactions (d) Dissolving of ammonium salts, such as Construct energy level diagrams for ammonium chloride, NH4Cl, ammonium endothermic reactions sulphate, (NH4)2SO4, in water. Interpret energy level diagram Laboratory Work 4.1: Exothermic and Interrelate energy change with endothermic reaction. formation and breaking of bonds Describe the application of knowledge 3. Carry out an activity to construct energy level of exothermic and endothermic diagrams for exothermic and endothermic reactions in everyday life. reactions.
4. Discuss to interpret an energy level diagram.
5. Discuss the release or the absorption of energy during formation and breaking of bonds using simulation, computer animation, games or other methods. 6. Show and discuss the application of exothermic and endothermic reactions, such as in cold or hot packs. Activity 4.2: Application of exothermic and endothermic reactions. Activity 4.3: Hot or cold pack.
17 Learning CCTS / TSTS / Learning Outcomes Activities Week Objectives SPS
22 4.2 A student is able to: 1. Discuss the meaning of heat of reaction for Making (21.6.10- Understanding State what heat of reaction is, the following types of reactions: hypothesis 25.6.10) heat of State what heat of precipitation is, (a) Precipitation, Controlling precipitation variables Determine the heat of precipitation for (b) Displacement, Experimenting a reaction, (c) Neutralisation (d) Combustion Communicating Construct an energy level diagram for Measuring and a precipitation reaction, 2. Carry out an activity to determine the heat of using numbers Solve numerical problems related to precipitation for a reaction and construct its Observing heat of precipitation energy level diagram. Interpreting data Laboratory Work: Heat of precipitation. Making inferences 3. Carry out an activity to solve numerical Comparing and problems related to heat of precipitation using contrasting information based on thermochemical Making equations. conclusion 23 4.3 A student is able to: 1. Discuss the meaning of heat of displacement Making (28.6.10- Understanding State what heat of displacement is hypothesis 2.7.10) heat of Determine heat of displacement 2. Carry out an activity to determine the heat of Controlling displacement variables Construct the energy level diagram displacement for a reaction and construct its Experimenting for a displacement reaction, energy level diagram. Laboratory Work 4.3: Heat of displacement Communicating Solve numerical problems related to Measuring and heat of displacement 3. Calculate heat of displacement using using numbers information based on thermochemical Observing equations. Interpreting data Making 4. Carry out an activity to solve numerical inferences problems related to heat of displacement Comparing and using information based on thermochemical contrasting equations. Making conclusion
18 Learning CCTS / TSTS / Learning Outcomes Activities Week Objectives SPS 24 4.4 A student is able to: 1. Discuss the meaning of heat of neutralisation. attributing (5.7.10- Understanding State what heat of neutralisation is, relating 9.7.10) heat of Determine the heat of neutralisation, 2. Carry out an activities to determine the heat of comparing and neutralisation Construct energy level diagrams for neutralization, and construct energy level contrasting various types of neutralisation diagrams, for the following types of reactions analyzing reactions, between: making Compare the heat of neutralisation for (a) Strong acid and strong alkali. inferences the reactions between a strong acid (b) Weak acid and strong alkali. visualizing and a strong alkali with the heat of (c) Strong acid and weak alkali. making analogies neutralisation for reaction between a (d) Weak acid and strong alkali. weak acid and/or weak alkali, Laboratory Work 4.4: Heat of neutralisation. Explain the difference between the Laboratory Work 4.5: Heat of neutralisation of heat of neutralisation for a strong acid acids and alkalis of different strenght. and/or strong alkali with heat of neutralisation for a reaction involving a 3. Discuss the difference between the heat of weak acid and a weak alkali. neutralisation for a strong acid and/or strong solve numerical problems related to alkali with heat of neutralization for a reaction heat of neutralization involving a weak acid and a weak alkali. 4. Carry out an activity to solve numerical problems related to heat of neutralisation using information based on the thermochemical equations.
25 4.5 A student is able to : 1. Discuss the meaning of heat of combustion. Attributing, (12.7.10- Understanding state what heat of combustion is, relating, 16.7.10) heat of determine heat of combustion for a 2. Carry outs activities to determine heat of observing, combustion reaction combustion of various alcohols. interpreting data, construct an energy level diagram for Experiment 4.1: Heat of combustion for alcohols. hypothesizing, a combustion reaction. making compare the heat of combustion of 3. Discuss: inferences, various alcohols (a) the difference between heat of controlling State what fuel values is combustion of various alcohols. variables, describe the difference between heat (b) The difference between fuel values of communicating, of combustion of various alcohols. various fuels. comparing and (c) The selection of suitable fuel for specific contrasting describe the applications of fuel value. purposes. compare and contrast fuel values for
19 Learning CCTS / TSTS / Learning Outcomes Activities Week Objectives SPS various fuels. solve numerical problems related to 4. Carry out an activity to solve numerical heat of combustion. problems related to heat of combustion using information based on thermochemical equations.
4.6 A student is able to: 1. Carry out group work where each group: Communicating Appreciating the . describe a variety of energy sources, (a) brainstorm and identify the various existence of . identify various technology used to (b) energy sources, various energy harness energy, (c) choose an energy source, sources . justify the use of a particular energy (d) identify technology used to harness source. (e) this energy, (f) d) discuss the pros and cons in using this (g) energy source.
2. Discuss the use of various energy sources and its effect on humans and the environment.
THEME : PRODUCTION AND MANAGEMENT OF MANUFACTURED CHEMICALS LEARNING AREA : 5. CHEMICALS FOR CONSUMERS
Learning CCTS / TSTS / Week Learning Outcomes Activities Objectives SPS 26 5.1 A student is able to: 1. Collect and interpret data on: Analysing (19.7.10- Analysing soap state what soap is, (a) the history of soap manufacturing. Being 23.7.10) and detergent state what detergent is, (b) what soap and detergent are, cooperative describe soap preparation process, (c) the additives in detergents such as describe detergent preparation biological enzymes whitening agents, Data interpreting process, (d) the preparation of detergent describe the cleansing action of soap, 2. Carry out an activity to prepare soap using the compare and contrast the saponification process effectiveness of the cleansing action Laboratory Work 5.1: Soap preparation process. of soap and detergent 3. Investigate the cleansing action of soap and detergent using simulation and computer
20 Learning CCTS / TSTS / Week Learning Outcomes Activities Objectives SPS animation 4. Discuss Experimenting identify the additives in detergent and (a) the cleansing action of soap and Sequencing their respective functions detergent Being honest and (b) The differences in the effectiveness of the accurate cleansing action of soap and detergent. Being responsible (c) Activity 5.1 Emulsifying property of soap Communication and detergent Activity 5.2: The cleansing action of soap and detergent. 5. Conduct a competition or carry out a project Differentiate related to Daring to try (a) The manufacturing of soap (b) The preparation of detergent for multiple purposes such as shampoo and dish cleaner.
27 5.2 A student is able to: 1. Collect and interpret data on the various types Analysing (26.7.10- Evaluating the state the types of food additives and of food additives in the market. 30.7.10) use of food their examples, Activity 5.6: Food additives Interpreting data additives state the function of each type of food 2. Collect and interpret data on the types of Comparing and additive, chemicals used in food additives and their contrasting justify the use of food additives, function as: describe the effects of food additives (a) preservatives and antioxidants, e.g. on health and the environment. sodium nitrate, sodium benzoate, Evaluating ascorbic acid, (b) flavouring agents, e.g. monosodium glutamate (MSG), aspartame, Making (c) stabilizers and thickening agents, e.g. conclusions gelatine, acacia gum, (d) dyes, e.g. azo compound, triphenyl compound. Generalising
3. Carry out a project to collect and observe the labels on food packs and identify the additives used. Activity 5.7: Food label.
21 Learning CCTS / TSTS / Week Learning Outcomes Activities Objectives SPS
4. Discuss: (a) the rationale for the use of food additives, (b) the effect of food additives on health and the environment, (c) life without food additives 28 5.3 A student is able to : 1. Collect and interpret data on various types Grouping and (2.8.10- Understanding state examples of traditional medicine, and functions, i.e : classifying 6.8.10) medicine their sources and uses, (a) traditional medicines derived from plants state the types of modern medicine and animals, Interpreting data and their examples, (b) analgesics such as aspirin, paracetamol state the functions of each type of and codeine, modern medicine, (c) antibiotics such as penicillin and describe the possible side effects of streptomycin, using modern and traditional medicine (d) psychotherapeutic medicine such as , stimulant, antidepressant and describe the correct usage of modern antipsychotic. and traditional medicines. Activity 5.8: Medicines Predicting, 2. Collect and interpret data on : Interpreting data (a) the side effects of modern and traditional medicines, (b) the correct usage of modern and traditional medicines. Activity 5.9: Drug abuse 29 5.4 A student is able to ; 1. Collect and interpret data on: Classifying (9.8.10- Appreciating the describe that the discovery of (a) discovery of chemicals that can improve Comparing and 13.8.10) existence of chemicals improves quality of life, the quality of life, such as antibiotic and contrasting chemicals state the side effects of chemicals on detergent, Attributing human and the environment, (b) side effects of chemicals on life and the describe common traits among environment, scientists in carrying out research, (c) describe common traits among scientists describe life without chemicals, in carrying out research, such as state appreciation and support for patience, meticulousness and proper management of chemicals. perseverance. Activity 5.10: The discovery of chemicals
2. Carry out an activity to discuss and predict
22 Learning CCTS / TSTS / Week Learning Outcomes Activities Objectives SPS how life would be without chemicals.
3. Discuss and practice proper management of chemicals towards better life, hygiene and health. 30 16.8.10- SPM STRATEGIK REVISION 20.8.10
MID TERM BREAK ( 22.8.09 – 30.8.09)
31-33 (18.8.10- 26.8.10 TRIAL EXAMINATION
34 (21.9.10- 25.9.10) CUTI HARI RAYA PUASA
35-41 (28.9.10-13.11.10) SPM STRATEGIC REVISION
SPM EXAMINATION 12.11.10 – 16.12.10)
SCHOOL HOLIDAY (20.11.2010 - 2.1.2010)
Prepared By: Checked By: Verified By:
Pn. Hjh Umaimah binti Harith Pn. Norlaila Abd Ghani Pn. Rohimah bt Md Amin Guru matapelajaran Guru kanan Sains PK Pentadbiran
23 24