Supporting Information:
Quantitative insights into the crystal structures of nitro derivatives of ethyl (2E)-2-cyano-3-phenylprop-2-enoate: Inputs from X-Ray Diffraction, DFT calculations and Hirshfeld Surface Analysis.
Dhananjay Dey a#, Venugopal Prakashb#, Deepak Chopra b*, Vasu c, Madnimaraiah Srinivas d
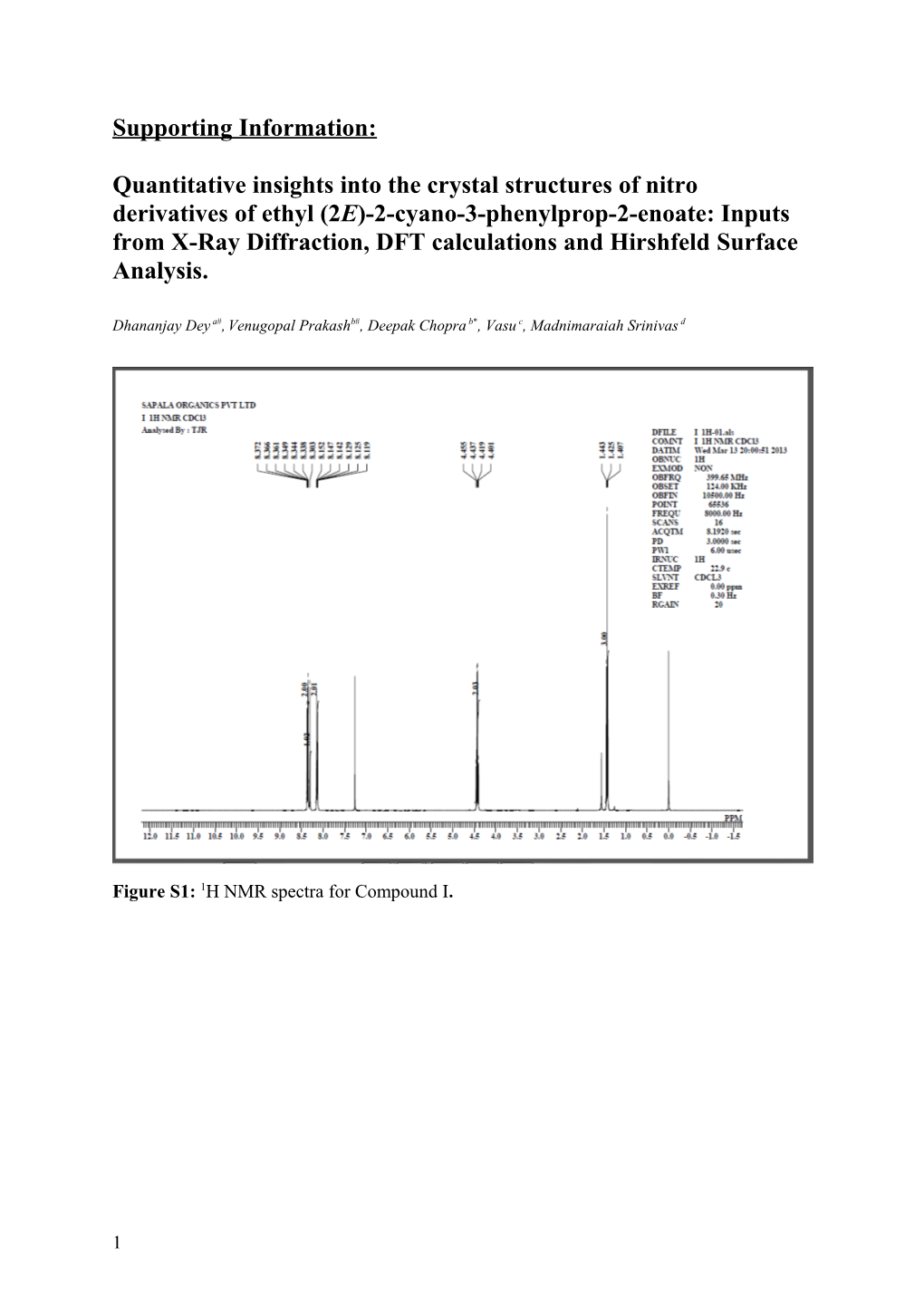
Figure S1: 1H NMR spectra for Compound I.
1 Figure S2: 1H NMR spectra for Compound II.
Fig S3: Experimental IR spectrum of compound I.
Figure S4: Overlay diagram of the synthesized molecules (I and II).
2 Table S1: Selected torsion angles (º) for I and II: Torsion Experimental (I, II) Theoretical (I, II) C4-C5-C7-C8 179.7(3), 179.2(3) 179.1a, 179.1a C3-C4-C5-C7 177.5(3), 175.7(3) 179.9a, 179.9a C2-O1-C3-C4 174.1(3), 174.5(3) 179.9a, 179.9a O2-C3-C4-C6 176.9(3), 179.1(3) 179.9a, 179.8a (a: Italicised values obtained from theoretical B3LYP/6-31G**calculation)
Table S2: Selected bond angles (º) for I and II: Bond angle Experimental (I, II) Theoretical (I, II) C3-C4-C6 116.0(3), 115.4(3) 117.4a , 118.0a C6-C4-C5 125.0(3), 125.5(3) 125.2a, 125.0a C4-C5-C7 131.2(3), 130.2(3) 131.9a, 131.8a (a: Italicised values obtained from theoretical B3LYP/6-31G**calculation)
Table S3: Intermolecular hydrogen bonds present in the synthesized molecules. I D-H...A D...H(Å) D...A(Å) H...A(Å) ÐD- Symmetry, Symmetry H...A(º) operation C1-H1B...O4 1.08 3.799(5) 2.75 163 x-1,-y+3/2, z-1/2,c-glide C1-H1B...O3 1.08 3.682(5) 2.97 125 x-1,-y+3/2, z-1/2,c-glide C8-H8...O2 1.08 3.421(5) 2.42 154 -x, -y+1, -z, i C5-H5...O2 1.08 3.443(5) 2.42 158 -x, -y+1, -z, i
C12-H12...N1 1.08 3.489(6) 2.79 123 -x, y+1/2, -z+1/2, 21
C11-H11...N1 1.08 3.497(6) 2.81 121 -x, y+1/2, -z+1/2, 21
C1-H1C...O4 1.08 3.788(6) 2.87 143 -x, y+1/2, -z+1/2, 21
C9-H9...O3 1.08 3.348(6) 2.30 163 -x+1, y-1/2, -z+1/2, 21 II D-H...A D...H(Å) D...A(Å) H...A(Å) ÐD- Symmetry H...A(º) C8-H8...O2 1.08 3.573(8) 2.61 149 -x+1, -y, -z+1, i C5-H5...O2 1.08 3.450(8) 2.41 161 -x+1, -y, -z+1,i C2-H2B...O3 1.08 3.728(10) 2.67 168 -x+2, -y, -z+1, i C2-H2A...O1 1.08 3.636(9) 2.77 138 x-1, y, z C11-H11...N1 1.08 3.412(9) 2.79 125 -x+3, -y+1, -z+1, i C12-H12...N1 1.08 3.397(9) 2.76 126 -x+3, -y+1, -z+1, i
3