Chapter 6
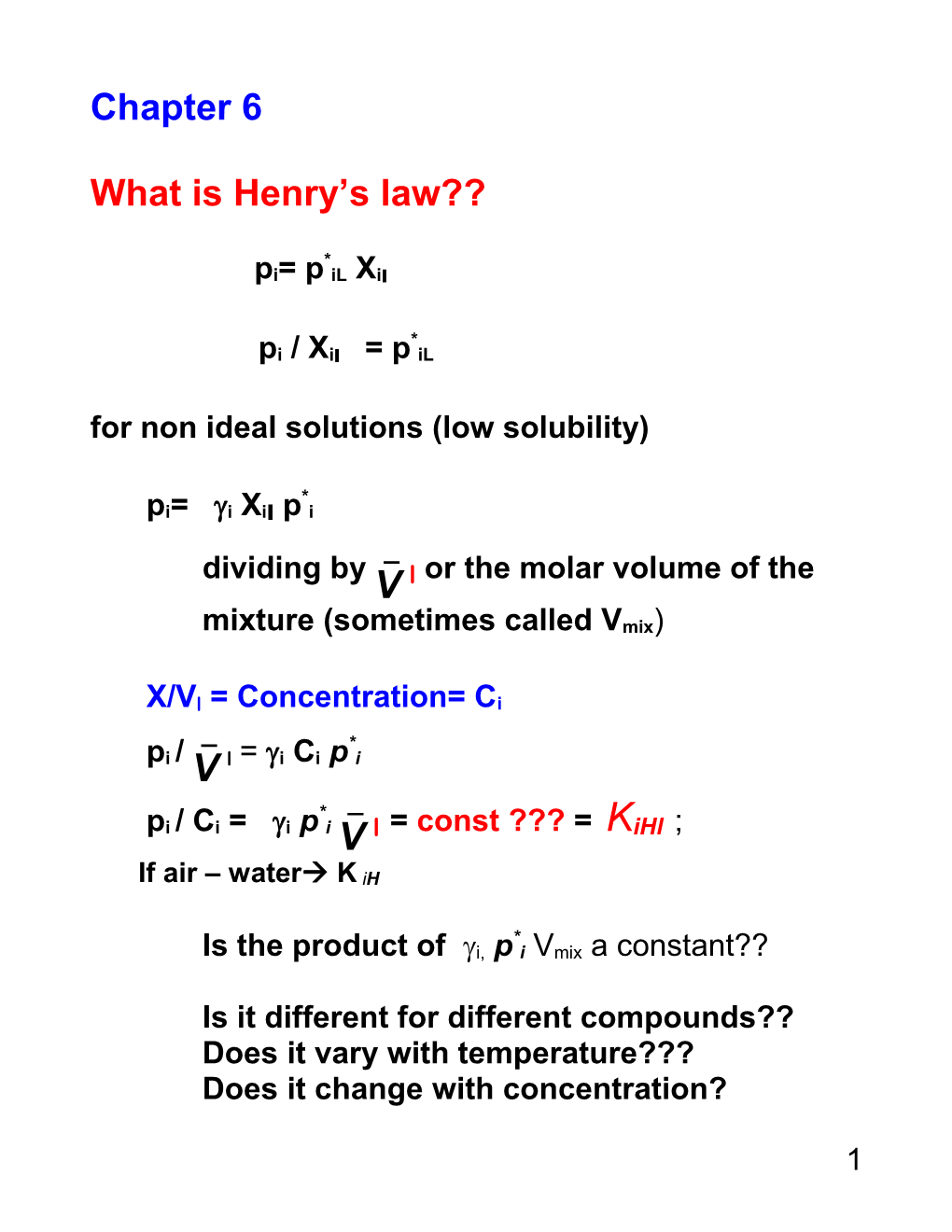
What is Henry’s law??
* pi= p iL Xil
* pi / Xil = p iL for non ideal solutions (low solubility)
* pi= i Xil p i _ dividing by V l or the molar volume of the mixture (sometimes called Vmix)
X/Vl = Concentration= Ci
_ * pi / V l = i Ci p i * _ pi / Ci = i p i V l = const ??? = KiHl ; If air – water K iH
* Is the product of i, p i Vmix a constant??
Is it different for different compounds?? Does it vary with temperature??? Does it change with concentration?
1 Does it change with salt or ionic content? How do we measure it?
Chapter 6 Henry’s Law
pi KiH Ciw traditionally
atmi 1 KiH 1 atmlitersmole molesliters w
Cia Kiaw (dimensionless Henry’s law const.) Ciw
Cia Cia KiH Cia KiH KiH Kiaw p n / V RT RT pi i KiH
2 If we go to the Appendix (p.1200, new book) and look at for Henry’s law values for air-water, we see -log Kiaw ;, * sat p iL and Ciw are referenced to their states.
how are these -log Kiaw values computed?
* sat Ideally, since KiH= pi / Ciw
If we go to a unit-less form, Kiaw, where Kiaw = KiH/RT
* sat So, log Kiaw= log { pi /RT } –log Ciw
For anthracene the Appendix has the following data:
* sat log pi = -3.01(Pa) -log Ciw = 6.60 -log Kiaw =2.8
1atm = 101,308 pascals
st * so 1 we need to get - log pi in atm
10-3.01 Pa/{ 101335 Pa/atm} = 9.646 x10-9 atm
* * * to change pi in atm. into Cair; pi V= nRT; Cair = pi /RT
R = 0.082 L atm./mole ; T = 298 K ; this gives -10 * Cair=3.94x10 moles/literair ; log pi /RT= -9.40
* sat -logKiaw= -log { pi /RT } +log Ciw
-logKiaw = 9.40 -6.60= 2.80 (and this is the book value)
3 o The old book is a lot cleaner; It gives -log p L and -log o p s directly in atmospheres
sat sat and –log Cw , -log Cs are in moles/liter and –log KH in liters atm/mol
So to get log KH in the appendix (p 621)of the old book for anthracene in (old book, p. 621)
o sat logKH = log pL – log Cw or
o sat logKH = log ps – log Cs for a liquid anthracene log KH = -6.11+ 4.48= -1.63 for a sold anthracene log KH = -8.1+6.46= -1.64
KH/RT = Kiaw (in new book); and -log Kiaw= 3.03
4 going back to Henry’s law
pi KiH Ciw
As Henry’s law values increase there is a tendency for higher gas phase concentrations over water i.e. partitioning is toward air for high vapor pressure compounds the fugacity in the gas phase is high
* fi = i Xifi pure liquid
* * (fi pure liquid = p i pure liquid)
High activity(i) coefs. favor partitioning to the gas phase i.e. Lower KiH and lower ‘s favor the liquid phase. Polar compounds?
5 Figure 6.2 page 111 (old book)
6 Wash out ratios or W and how fast does the atmosphere clean up during a rain Usually defined as the conc. in rain/conc. In air
W = Ciw/Cia = 1/Kiaw
W x Cia = conc in the rain, Ciw , with units of moles/ cc water or Ciw in units of moles i /cc = moles i/g H20
The rain can be viewed as a flux and has an intensity I, with units of grams of rain sec-1 cm-2 so now
-1 -2 ------I x Ciw = g rain sec cm x moles i/g H20
Since W = Ciw/Cia = 1/Kiaw and Ciw = 1/Kiaw x Cia
I x 1/Kiaw x Cia = moles of i from the atmosphere hitting the surface of the earth in the rain per sec-1 cm-2 And this is a flux too
7 We will learn in Chapter 20,
Flux / (conc x depth ) = 1st order rate constant in
A = Ao e-kt
So if you know the rain intensity, Kiaw and the height of the atmosphere, you can estimate how fast the atmosphere will “clean” up with a given rain intensity??? ______
Flux = I x 1/Kiaw x Cia = moles of i from the atmosphere hitting the surface of the earth per sec-1 cm-2
If the mixing height of the atmosphere is 300 m and we have a rain that gives an 1” of water in 2 hours I = 2.5g cm-2 /(2x60x60 sec) = 3.47x10-4 g cm-2 sec-1
-5 Kiaw phenol = 2x10
krate constat = I x 1/Kiaw x Cia / (Cia x30,000 cm) in units of 1/sec
8 in units of 1/sec = 0.00059 sec-1
C/Co = e-kt ; t = 2 hours = 2x60x60 sec
C/Co = 0.0145 or 98.5% of the phenol will be cleaned out in the air in the rain
How do different Henry’s law values impact this calculation?
9 Concentration effects on KiH
Ciw = Xi / Vw Vw = molar vol. H2O
* pi iw iw pl * KiH iw pl Vw Ciw iw /Vw
Under dilute conditions KiH is directly proportional to the:
activity coef. saturated vapor pressure molar volume of water
10 What is the effect of concentration on KiH? * P ia water
organic
* at saturation the vapor pressure pi = p iw
* pi = i Xi p i pure liquid
sat sat 1 pi 1 X iw sat * sat iw pi iw
psat sat i sat * K pl Vw iH Csat w iw
sat The question becomes how does KiH differ from KiH ?
11 If the activity coef. changes with increases in sat concentration of Ciw then KH will change?
Why?
The old book suggests from benzene partitioning data, sat that little difference may exist between KiH and KiH. For benzene K’iaw = (Cair/Ciw) a difference of <4% was observed between saturated and dilute water solutions….
This means that KiH can sometimes be approx. from psat sat sat i KiH and estimated from K iH Csat iw
Example
sat -3 o If the Ciw for chlorobenzene = 4.3x10 mol/L at 25 C
* -2 and p iL = 1.6x10 atm what is the KiH
psat 2 sat iL 1.6x10 atm KiH K 3.6atm L / mol iH Csat 4.3x103 mol / L iw
12 K 3.6atm L mol 1 K iH 0.15 iaw RT 0.082atm L mol 1K 1x298K
sat A simple way of changing iw into iw (this does not always work)
log sat log i i sat 2 (1 xi )
psat K sat iw sat p* V iH sat w iL w Ciw for infinitely dilute solutions
pi iw iw p *iL * KiH iw piLVw Ciw iw / Vw
13 sat Comparison of iw and iw
sat sat sat iw -logCiw Ciw iw
(Tab 5.2) (p618) mol/L 1/(CsatVmix) (old book) benzene 2400 1.64 0.0229 2425 toluene 12000 2.25 0.0056 9879 chlorobenz 19000 2.35 0.00447 12437 hexCl-benz 9.8E+8 5.56 2.75E-6 2.0E+7 octanol 37000 2.35 0.00447 18656
sat Why are iw values sometimes greater than iw ?
14 Effect of Temperature
* vapHi 1 ln piL const R T by analogy E H 1 ln x sat iw const iw R T
sat sat xiw Ciw so substituting Vmix excess heat of solution E H 1 lnCsat iw const w R T (Vmix )
* PiiL KiH sat CIw
E sat H H ln K VAP i iw const + iH RT H
15 E page 115, Table 6.1 vapHi- H iW = awHiHHenry
16 Figure 6.3 page 116 (old book)
17 What are the effects of salts? in Chapter 5 the relationship between a saturated solution in water vs. sea water is discussed
(Setschenow, 1889)
sat Ciw s log sat K i [ salt ]tot Ciw ,salt let’s say we want to calculate the equilibrium distribution of anthracene in sea water, ie KiH w,salt if we transform Setschenow’s equation
sat sat s logC iw ,salt logCiw Ki [ salt ]tot
s sat sat Ki [ salt ]tot C iw ,salt Ciw 10
the Henry’s law for salt water is * * p s iL piL sat Ki [ salt ] KiH ,w ,salt sat s KiH 10 C sat Ki [ salt ] iw ,salt Ciw 10 s for anthracene Ki = 0.3, assume [salt] = 0.5 M 18 and KiH = 0.078 atm L mol-1 (0.3)x(0.5) -1 so KIH,w,salt= 0.078x10 =0.11 atm L mol
19 Table 6.2 p 117 (old book)
20 Estimating Henry’s Law values
Hine and Mookerjee (1975)
Log Kiaw =nj x functional groupi OH for phenol there are
(old book) (new book) p 206 Table 6.2 6 aromatic carbons at: -.33/carbon -0.264 5 aromatic C-H groups, at: 0.21/group +0.154 and one C-O group at: 0.74 -0.596 (C-OH) and one OH group at: -3.21 -3.232
(old book) log K’H = 6x(-.33)+5(.21)+0.74+(-3.21) = -3.40
(New book) log Kiaw= -4.64
K’H = 0.0004 ; new book Kiaw= 0.000023
sat from p*iL / C w= 0.00041
21 22 From Chapter 3 (Discontented Socrates) There are linear free energy techniques that permit the estimation of equilibrium constants base on molecular structure This is possible if one assumes that the overall free energy of phase transfer is related to the linear combinations of the free energies related to the individual parts of the molecule that are involved in the transfer.
12Gi = 12Gparts of i + special interaction terms
LogK i12 = LogKparts of i12 + special interaction terms
Returning to main body of the lecture discuss a technique that is often used in the book Mass= concentration x volume
We often become interested in the fractional mass in one phase
23 Example Problem: Consider a well sealed flask with 100 ml of H2O and 900 ml air. At equilibrium estimate the amount of chlorobenzene in the air and in the water if the sum (total) in both phases is 10 g. fw = the fraction in the water phase fw = chlorobenzene mass in water/total mass
C V 1 1 f iw iw w C V V CiwViw CiaVia ia ia ia 1 1Kiaw CiwViw Viw
Using the Hine and Mookerjee
Cl K’H = Kiaw= 0.1622
fw = 1/{(1+0.1662)900/100}=0.41 the concentration in the aqueous phase Ciw is
Ciw = fiw Mtot / Viw
Ciw = 0.41x10g /0.1L = 41 g/Lwater
Cia= 0.59x10g /0.9 L = 6.6g/Lair
24 Experimental Measurements 1. air toluene McAuliffe (1971)
CiwVwv fract in H2O = CiaVg CiwVwv
Vwv = vol of water for dilute systems
Kiaw= Cia/Ciw = Dg,w( a gas/water part. coef.)
Vwv fract in H2O= const. KiawVg Vwv
For then next time step,
If Vg and Vwv are equal;
Ciw,1 = Ciw,o x fract in H2O;
25 because Ciw,1 = Ciw,o x Ciw,1/(Ciw,1+Cai,1)
n Hence Cia,n= (fact in H2O) Ciw,o Kiaw taking the logs of both sides and substituting for fract in H2O and remembering that Kiaw=Dgw
V wv +log (C D logCia,n n log iw o gw) Kiaw Vg Vwv
Figure 6.4 page 119 (old book)
26 2. Mackay and co-workers experimental KH technique using a stripping apparatus
o C w initially
bubbles
IF we take this as a CSTR the conc. of Cw some time, t, after we start the bubbles is
o -kt Cw = C w e if we were just flowing in clean water instead of bubbles into some volume of water Vw
o -f/Vw t Cw = C w e
Cw time to take into account the gas that is stripping, f, the flow of water is replaced with
Kiaw x flow rate bubbles *
o - Kiaw V /Vw t Cw = C w e g
27 Table 6.3, p120
28 An acid Rain example
Atmospheric acidity of “pure” rain
CO2 +H2O --> CO2H2O (1)
for reaction 1, KiH(CO2) = pCO2 / CO2H2O
CO2H2O dissociates in water
+ - CO2H2O + H [HCO3 ] (2)
the equilibrium const K2 for this reaction is:
- + - + [HCO3 ] [H ] [HCO3 ] [H ] KHCO2 K2 = =
CO2H2O pCO2 bicarbonate reacts to
- -2 + HCO3 CO3 +H (3)
-2 + -2 + [CO3 ] [H ] [CO3 ] [H ] KH(CO2) K3 = = - [HCO3 ] K2 pCO2
29 we now have expressions for each carbon form and we -2 - could add up [CO3 ] + HCO3 ]+ [CO2H2O] and set this to [CO2]T
p CO2 K1 K1K2 [CO2 ]T 1 K 2 HCO2 [H ] [H ]
An additional condition of ions in solution is that there be electrical neutrality, ie.
- - -2 [H+] = [OH ]+[HCO3 ]+2[CO3 ]
H2O H OH 0)
- + [HCO3 ] [H ] KH(CO2) K2 = 2) pCO2
-2 + [CO3 ] [H ] KH(CO2) K3 = 3) K2 pCO2
If we substitute for each of the ions in the electro-neutrality equation 30 K K p 2K K K p [K ] H(CO 1 CO2 H(CO 1 2 CO2 [H+ ] W 2) 2) [H+ ] [H+ ] [H+ ]2
AT a given temperature, KH(CO2) K1,K2 Kw are known. For pCO2 = 330 ppm, it can be shown that at 283oK the pH will be ~5.6. This is often the value cited for “pure” rain water.
The above equation can be numerically solved in most spread sheets!, by moving the [H+] on the left to the right side of the equation.
31 Using fugacities to model environmental systems (Donald Mackay ES&T, 1979) Consider the phase equilibrium of five environmental compartments. Is it possible to tell where an environmental pollutant will concentrate? A
B C E D where A= air, B= lake, C= Soil, D= Sediment, E= biota and suspended solids
When a system is at equilibrium the escaping tendencies in each phase are equal
fA = fB = fC = fD = fE
32 For Example: oxygen in water at 0.3 mol/m3 and in air at 8mol/m3 exert the same escaping tendency of 0.2 atm and are thus in equilibrium with the same fugacity.
1. Fugacitys are linearly related to conc. oxygen in water at 0.03 mol/m3 exerts a fugacity of one tenth the fugacity of 0.3mol/m3.
fA = fB = fC = fD = fE
Fugacities can be translated into concentrations
fi Zi = Ci where Z is called a fugacity capacity value ------
3. The mass Mtotal = Ci Vi = fi Zi Vi
if the system is at equilibrium
Mtotal = fi Zi Vi
Mi = fi Zi VI
4. Calculating Z values
33 Zi fi = Ci; Zi= C/f
In air f is equal to the partial pressure, pi
piV = nRT, so fi = pi = Cair RT, so Ziair = 1/RT
at 298K , RT= 0.082 liter atm K-1 mol-1x298K
RT= 0.025 m3 atm mol-1
------
In water pi = KiH Ciw and Ciw = Z fiw
pi = KiH Ziw fiw and we said in air pi = fia
so Ziw = pi /{fiw KiH}= 1/KiH
We will use a representative value of -4 3 -1 KiH= 1x10 m atm mol
34 On soils, sediment, and suspended solids
Cwi + S ----> Cis
CiS Kid = KiwS ; Cis = KiwsxCiwxS CiW xS
Cis =Zi sp x fis and Ciw = pi /KiH
Zi sp = KiwS x 1/KiH x pi x S/fis = Ki wS x S/ KiH
For suspended solids at 1,000 mg/m3 and -4 3 3 -1 -3 a Ki sp of 10 m /mg, Zsp= 10 mol atm m
For sediment and soils at 2x109 mg/m3 and -5 3 9 -1 -3 a Kid = Kiws of 5x10 m /mg, Zs,s= 10 mol atm m
For Aquatic Biota
ZB = B y Kiow/KiH
-6 3 3 where B is the volB/vol H20= 5x10 m /m ,
5 4 y=octanol fract. of B = 0.2, Kiow=10 ; ZB=10
------
35 Let’s look at the Equilibrium Distribution of a toxic compound with an atmospheric concentration of 4 x 10-10 mol/m3. (fi x Zi = and Mi = fi Zi Vi)
Z Vol fi M % g/m3. (m3) (atm) (moles) air 40 1010 10-11 4 0.35 water 104 106 10-11 10-1 0.01 10-5 s solids 103 106 10-11 10-2 0.001 0.01 Sed 109 104 10-11 102 9.1 0.05 Soil 109 105 10-11 103 90.5 0.5 Aq biota 104 106 10-11 10-1 0.01 0.2
36 Chapter 6 fugacity homework problem 3 3. A Fugacity problem (first some additional theory )
-3 - In a compartment, Ci, the rate of decay in moles m year 1 for a given process, j, is
rateCi = kj[Ci]
where kj = bio degradation photolysis hydrolysis oxidation advection etc.
rateCi= kB[Ci]+ kP[Ci]+ kH[Ci]+ kOX[Ci]+ kA[Ci]
rateCi = [Ci] kj = [Ci] kT in moles per year the total rate in a compartment of volume Vi is:
rateTi = [Ci] kT Vi if the system is at steady state in each compartment, the total input rate for all the compartments in moles/ year will equal the amount reacted in moles/year
37 I = Ci]kT Vi) = Zi fi kT Vi) and
I = fi Zi kT Vi); why? so
fi= I /Zi kT Vi)
Assume three compartments: air, water and sediment (sed) in equilibrium with one another and a total input rate of 1000 moles/year goes into the entire system. From the following rate constant data (years-1), calculate for each compartment the fugacity, the resulting concentration (moles/m3), the total decay rate (moles/year) and the total #moles in each compartment. Use Z values and volumes from the class example. From the total decay rate, where does most of the degradation take place? where does most of the mass end up? Use a spread sheet
Rate constants (years-1) Biodeg. photolysis hydrolysis oxidation advection kT
Air 0 130 0 0 50 180 water 100 100 100 0 200
Sed. 0.1 0 0 0 0
38