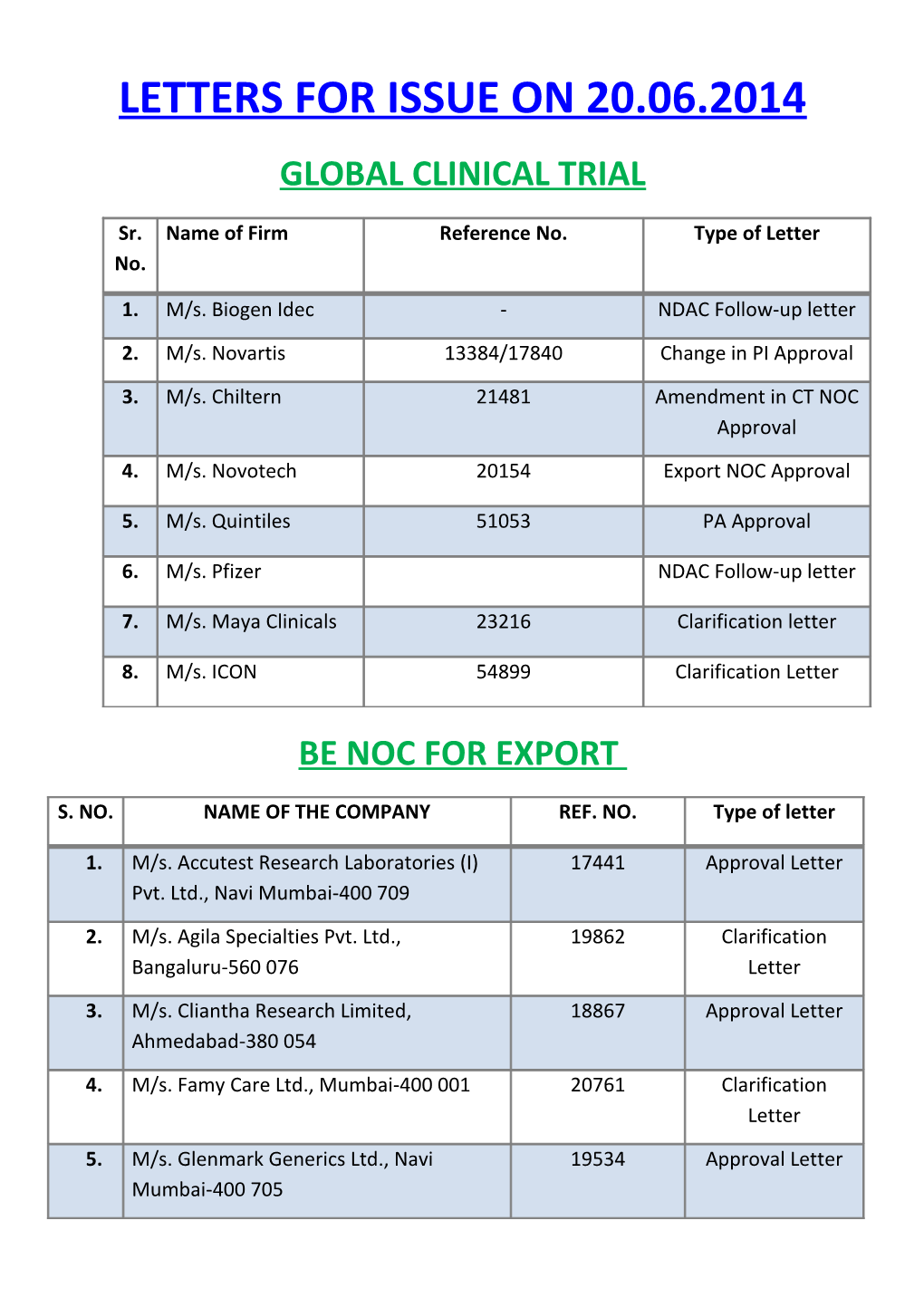
LETTERS FOR ISSUE ON 20.06.2014 GLOBAL CLINICAL TRIAL
Sr. Name of Firm Reference No. Type of Letter No.
1. M/s. Biogen Idec - NDAC Follow-up letter
2. M/s. Novartis 13384/17840 Change in PI Approval
3. M/s. Chiltern 21481 Amendment in CT NOC Approval
4. M/s. Novotech 20154 Export NOC Approval
5. M/s. Quintiles 51053 PA Approval
6. M/s. Pfizer NDAC Follow-up letter
7. M/s. Maya Clinicals 23216 Clarification letter
8. M/s. ICON 54899 Clarification Letter
BE NOC FOR EXPORT
S. NO. NAME OF THE COMPANY REF. NO. Type of letter
1. M/s. Accutest Research Laboratories (I) 17441 Approval Letter Pvt. Ltd., Navi Mumbai-400 709
2. M/s. Agila Specialties Pvt. Ltd., 19862 Clarification Bangaluru-560 076 Letter
3. M/s. Cliantha Research Limited, 18867 Approval Letter Ahmedabad-380 054
4. M/s. Famy Care Ltd., Mumbai-400 001 20761 Clarification Letter
5. M/s. Glenmark Generics Ltd., Navi 19534 Approval Letter Mumbai-400 705 6. M/s. Glenmark Generics Ltd., Navi 19533 Approval Letter Mumbai-400 705
7. M/s. Inventia Healthcare Pvt. Ltd., Dist. 19527 Approval Letter Thane-421 506
8. M/s. Lambda Therapeutic Research Ltd., 12351 Additional Study Ahmedabad-380 061 Centre
9. M/s. Lotus Labs Pvt. Ltd., Chennai-600 21229 Approval Letter 004
10. M/s. Micro Therapeutic Research Labs 13023 Amendment Pvt. Ltd., Coimbatore-641029 Letter
11. M/s. Quest Life Sciences (P) Ltd., 20862 Approval Letter Chennai-600045
12. M/s. Semler Research Center Pvt. Ltd., 14953 Clarification Bangalore-560078 Letter
13. M/s. Sun Pharmaceutical Industries Ltd., 16636 Clarification Vadodara-390 020 Letter
14. M/s. Sun Pharmaceutical Industries Ltd., 19014 Approval Letter Vadodara-390 020
15. M/s. Sun Pharmaceutical Industries Ltd., 19019 Approval Letter Vadodara-390 020
16. M/s. Synchron Research Services Pvt. 18516 Amendment Ltd., Ahmedabad-380 054 Letter
17. M/s. Unichem Laboratories Ltd., Mumbai- 48911 Approval Letter 400 102
18. M/s. Veeda Clinical Research Pvt. Ltd., 22325 Approval Letter Ahmedabad-380 015
19. M/s. Vimta Labs Ltd., Hyderabad-500051 20234 Approval Letter
20. M/s. Watson Pharma Pvt. Ltd., Navi 19460 Clarification Mumbai-400 614 Letter
21. M/s. Wockhardt Limited, Aurangabad- 60283 Approval Letter 431 006 ONLY TEST LICENCE FOR BE
S. NO. NAME OF THE COMPANY REF. NO. Type of letter
1. M/s. Accutest Research Laboratories (I) Pvt. 19819 Test Licence Ltd., Navi Mumbai-400 709
2. M/s. Cliantha Research Limited, Ahmedabad- 18868 Test Licence 380 054
3. M/s. Cliantha Research Limited, Ahmedabad- 17711 Test Licence 380 054
4. M/s. Emcure Pharmaceuticals Limited, Pune- 20255 Test Licence 411 026
5. M/s. GVK Biosciences Pvt. Ltd., Hyderabad-500 23030 Test Licence 038
6. M/s. Kusum Healthcare Pvt. Ltd., New Delhi- 25433 Test Licence 110 020
7. M/s. Lambda Therapeutic Research Ltd., 13699 Test Licence Ahmedabad-380 061
8. M/s. Lotus Labs Pvt. Ltd., Bangalore-560 034 20640 Test Licence
9. M/s. Om Sai Clinical Research Pvt. Ltd., Sangli- 24884 Test Licence 416 416, Maharsahtra
10. M/s. Vimta Labs Ltd., Hyderabad-500051 19955 Test Licence
11. M/s. Watson Pharma Private Ltd., Navi 18446 Test Licence Mumbai-400 614
12. M/s. Watson Pharma Pvt Ltd ., Navi Mumbai- 19949 Test Licence 400614
GLOBAL CLINICAL TRIAL
Sr. Name of Firm Reference No. Type of Letter No. 1. M/s. Biogen Idec 7521/17358 Clarification Letter
2. M/s. Quintiles 4604 Clarification Letter
MEDICAL DEVICE
S.NO. COMPANY NAME DIARY NO STATUS
1. M/s. Nutri Synapzz Therapeutix Private Limited. 20628 Clarification Letter
2. M/s. White Pharmaceuticals. 20861 Clarification Letter
3. M/s. Mais India Medical Devices Pvt. Ltd. 16022 Query Letter (CLAA)
The State Drugs Controller, Haryana (CLAA)
TEST LICENCES
S. No. Name of the Company Diary No. Type of Letter
1. Actavis Pharma Development 21465, Approval Letter Centre Pvt. Ltd 32013
2. Actavis Pharma Development 21466, Approval Letter Centre Pvt. Ltd 32018
3. Actavis Pharma Development 21467, Approval Letter Centre Pvt. Ltd 32023
4. Alembic Pharmaceuticals Ltd 21521, Approval Letter 31933
5. APL Research Centre 21336, Approval Letter 31773
6. Cipla Ltd 21657, Approval Letter 32286
7. Cipla Ltd 21658, Approval Letter 32282
8. Cipla Ltd 21659, Approval Letter 32285
9. Cipla Ltd 21660, Approval Letter 32279
10. Cipla Ltd 21661, Approval Letter 32277
11. Cipla Ltd 21662, Approval Letter 32328
12. Fresenius Kabi Oncology Ltd 16262, Approval Letter 24265, 23360, 34885
13. Getz Pharma Research Pvt. Ltd 21582, Approval Letter 32264
14. Getz Pharma Research Pvt. Ltd 21583, Approval Letter 32266
15. Getz Pharma Research Pvt. Ltd 21584, Approval Letter 32213
16. Getz Pharma Research Pvt. Ltd 21585, Approval Letter 32217
17. Getz Pharma Research Pvt. Ltd 21587, Approval Letter 32258
18. Glenmark Pharmaceuticals Ltd 21533, Approval Letter 31959
19. Glenmark Pharmaceuticals Ltd 21534, Approval Letter 31965
20. Glenmark Pharmaceuticals Ltd 21535, Approval Letter 31970
21. Glenmark Pharmaceuticals Ltd 21536, Approval Letter 31973 22.
23. Intas Pharmaceuticals Ltd 21593, Approval Letter 32273
24. Intas Pharmaceuticals Ltd 21594, Approval Letter 32272
25. Ipca Laboratories Limited 21471, Approval Letter 32040
26. Ipca Laboratories Limited 21487, Approval Letter 31900
27. Ipca Laboratories Limited 21488, Approval Letter 31905
28. Ipca Laboratories Limited 21489, Approval Letter 31909
29. Ipca Laboratories Limited 21490, Approval Letter 31916
30. Ipca Laboratories Limited 21491, Approval Letter 31920
31. Ipca Laboratories Limited 21492, Approval Letter 31927
32. Lupin Ltd 16110, Approval Letter 24119, 24096, 35600
33. Mylan Laboratories Ltd 21572, Approval Letter 32227
34. Novartis Healthcare Pvt Ltd 8644, Approval Letter 13075, 20373, 30575, 22872, 33751
35. Novartis Healthcare Pvt Ltd 13708, Approval Letter 21391, 22873, 33932
36. Novartis Healthcare Pvt Ltd 13676, Approval Letter 21166, 22873, 33932
37. Novartis Healthcare Pvt Ltd 13709, Approval Letter 21388, 22873, 33932
38. Novartis Healthcare Pvt Ltd 13679, Approval Letter 21170, 22873, 33932
39. Pfizer Products India Pct Ltd 9647, Approval Letter 14980, 15209, 22959
40. Pfizer Products India Pct Ltd 9648, Approval Letter 14956, 15210, 22955
41. RA Chem Pharma Ltd 12404, Approval Letter 19243, 20243
42. Ranbaxy Laboratories Ltd 21612, Approval Letter 32289
43. Ranbaxy Laboratories Ltd 12329, Approval Letter 18670, 22317, 33307
44. Reliance Life Sciences Pvt Ltd 16079, Approval Letter 24173, 20334, 36488
45. Roche Products (India) Pvt Ltd 17055, Approval Letter 25172, 19878, 29791, 24620, 36015
46. Sequent Research Ltd 21422, Approval Letter 32059
47. Sequent Research Ltd 21423, Approval Letter 32069
48. Shalina Laboratories Pvt Ltd 21524, Approval Letter 31943
49. Shalina Laboratories Pvt Ltd 21525, Approval Letter 31950
50. Shasun Pharmaceuticals 21573, Approval Letter Limited 32237
51. Shasun Pharmaceuticals 21574, Approval Letter Limited 32249
52. Stabicon Life Sciences Pvt Ltd 21233, Approval Letter 31858
53. Stabicon Life Sciences Pvt Ltd 21234, Approval Letter 31860
54. Sun Pharma Advanced 21591, Approval Letter Research Company Ltd 32274
55. Sun Pharmaceutical Industries 21589, Approval Letter Ltd 32255
56. Unichem Laboratories Ltd 21486, Approval Letter 32034
57. United States Pharmacopeia- 21436, Approval Letter India (P) Ltd 32120 58. United States Pharmacopeia- 21437, Approval Letter India (P) Ltd 32118
59. United States Pharmacopeia- 21438, Approval Letter India (P) Ltd 32117
60. United States Pharmacopeia- 21439, Approval Letter India (P) Ltd 32116
61. United States Pharmacopeia- 21459, Approval Letter India (P) Ltd 32112
62. United States Pharmacopeia- 21460, Approval Letter India (P) Ltd 32107
63. United States Pharmacopeia- 21461, Approval Letter India (P) Ltd 32121
64. V-ensure Pharma Technologies 21609, Approval Letter Pvt. Ltd 32268
65. Watson Pharma Pvt. Ltd 21470, Approval Letter 32028
66. Wintac Ltd 14436, Approval Letter 21919, 21419, 32063
67. APL Research Centre 18703, Deficiency 27933 Letter
68. Cipla Ltd 20060, Deficiency 30326 Letter
69. Getz Pharma Research Pvt Ltd 21318, Deficiency 31582 Letter
70. Ipca Laboratories Ltd 20623, Deficiency 30732, Letter 20626, 30889, 20627, 30886 71. Jubilant Life Sciences Ltd 20827, Deficiency 31034 Letter
72. Modi Mundipharma Pvt Ltd 21424, Deficiency 32122 Letter
73. RA Chem Pharma Ltd 20242, Deficiency 30102 Letter
74. Sanofi-Synthelabo (India) Ltd 20709, Deficiency 31031, Letter 20710, 31027, 20712, 30966, 20713, 30962, 20714, 30654, 20716, 30657, 20930, 31168, 20942, 31181, 20943, 31308, 20944, 31306
75. Shilpa Medicare Ltd 17182, Deficiency 25478 Letter
76. Stabicon Life Sciences Pvt Ltd 21235, Deficiency 31862, Letter 21238, 31732, 21238-A, 31729,
77. Stabicon Life Sciences Pvt Ltd 21095, Deficiency 31723 Letter
78. Vimta Labs Ltd 20986, Deficiency 31082 Letter