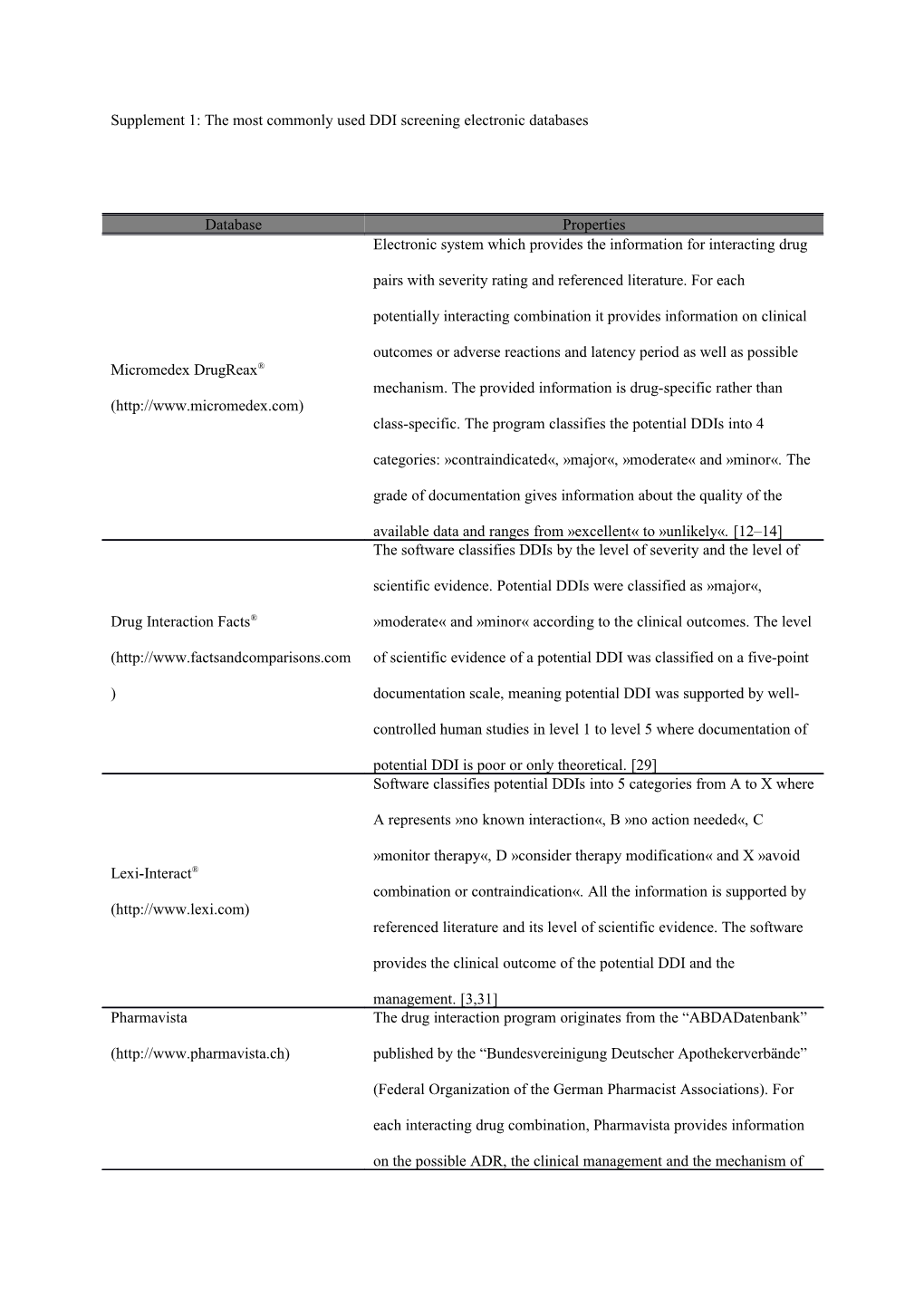
Supplement 1: The most commonly used DDI screening electronic databases
Database Properties Electronic system which provides the information for interacting drug
pairs with severity rating and referenced literature. For each
potentially interacting combination it provides information on clinical
outcomes or adverse reactions and latency period as well as possible Micromedex DrugReax® mechanism. The provided information is drug-specific rather than (http://www.micromedex.com) class-specific. The program classifies the potential DDIs into 4
categories: »contraindicated«, »major«, »moderate« and »minor«. The
grade of documentation gives information about the quality of the
available data and ranges from »excellent« to »unlikely«. [12–14] The software classifies DDIs by the level of severity and the level of
scientific evidence. Potential DDIs were classified as »major«,
Drug Interaction Facts® »moderate« and »minor« according to the clinical outcomes. The level
(http://www.factsandcomparisons.com of scientific evidence of a potential DDI was classified on a five-point
) documentation scale, meaning potential DDI was supported by well-
controlled human studies in level 1 to level 5 where documentation of
potential DDI is poor or only theoretical. [29] Software classifies potential DDIs into 5 categories from A to X where
A represents »no known interaction«, B »no action needed«, C
»monitor therapy«, D »consider therapy modification« and X »avoid Lexi-Interact® combination or contraindication«. All the information is supported by (http://www.lexi.com) referenced literature and its level of scientific evidence. The software
provides the clinical outcome of the potential DDI and the
management. [3,31] Pharmavista The drug interaction program originates from the “ABDADatenbank”
(http://www.pharmavista.ch) published by the “Bundesvereinigung Deutscher Apothekerverbände”
(Federal Organization of the German Pharmacist Associations). For
each interacting drug combination, Pharmavista provides information
on the possible ADR, the clinical management and the mechanism of the pDDI, and provides literature references regarding the pDDI.
The program classifies severities of pDDIs into five categories: major,
moderate, minor, insignificant, and unidentified source. [3,33] A freely available online medical decision support tool which screens
for potential DDIs and classifies them according to mechanism as
Epocrates Rx® pharmacokinetic, pharmacodynamic or unknown. Potential DDIs are
(http://www.epocrates.com) classified into categories by management strategy as »contra-
indicated«, »avoid combination/use alternative«, »modify
treatment/monitor« and »caution«. [34] Electronic database which features detailed comments on more than
20000 drug interactions involving about 2000 specific substances. It
MediQ classifies the potential DDIs according to severity into 4 categories. It
also provides free texts for each DDI and phamacokinetic and
pharmacodynamic effects. [35] The software is available on internet and provides classification Drug Interaction Checker according to severity of potential DDIs into 3 categories: major, (http://www.drugs.com) moderate and minor. [36]