MR. SURRETTE VAN NUYS HIGH SCHOOL
CHAPTER 11: THE ATOM AND THE NUCLEUS CLASS NOTES
ELEMENTS An element is any material that cannot be broken down by chemical means into simpler materials. Hydrogen, carbon, nitrogen, and the rest of the periodic table are all elements. An atom is the smallest part of an element.
ELECTRONS Electrons are sub-atomic particles that occur outside the nucleus. They have a charge of -1 and a mass of 9.1 x 10-30 kg.
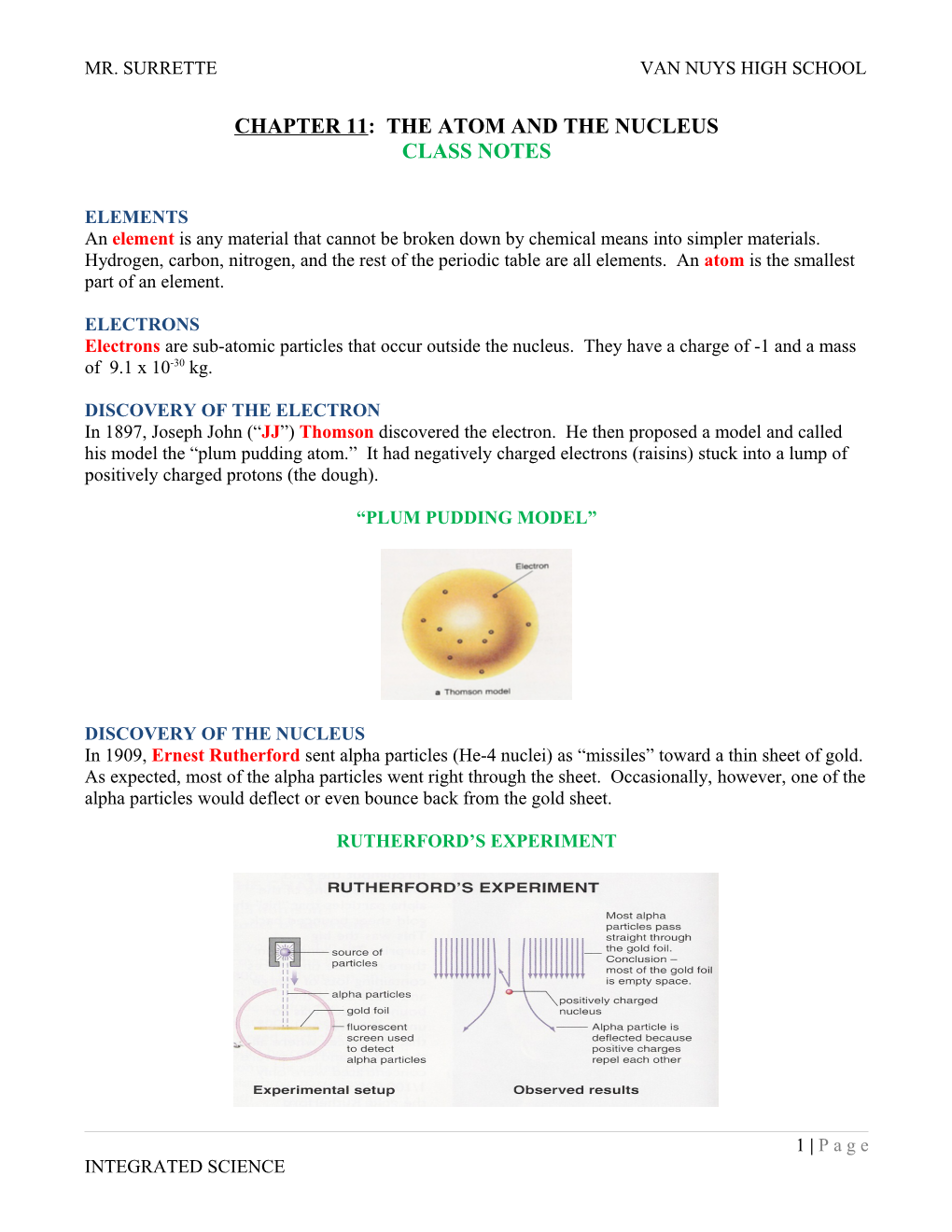
DISCOVERY OF THE ELECTRON In 1897, Joseph John (“JJ”) Thomson discovered the electron. He then proposed a model and called his model the “plum pudding atom.” It had negatively charged electrons (raisins) stuck into a lump of positively charged protons (the dough).
“PLUM PUDDING MODEL”
DISCOVERY OF THE NUCLEUS In 1909, Ernest Rutherford sent alpha particles (He-4 nuclei) as “missiles” toward a thin sheet of gold. As expected, most of the alpha particles went right through the sheet. Occasionally, however, one of the alpha particles would deflect or even bounce back from the gold sheet.
RUTHERFORD’S EXPERIMENT
1 | P a g e INTEGRATED SCIENCE MR. SURRETTE VAN NUYS HIGH SCHOOL
THE NUCLEUS AND THE PROTON Rutherford concluded that almost all the mass and positive charge of the atom is concentrated in a dense center, which he called the nucleus. He also defined the proton as the smallest unit of positive charge inside the nucleus.
ATOMIC NUMBER The number of protons Z in the nucleus is called the atomic number.
NUCLEONS Neutrons are neutral particles within the nucleus that hold the nucleus together. Both protons and neutrons are called nucleons because they reside in the nucleus.
ATOMIC MASS The number of protons Z plus the number of neutrons N is the atomic mass A:
A = Z + N
MODEL OF THE ATOM
ISOTOPES Atoms of the same element with different numbers of neutrons are called isotopes. Isotopes are identified by their mass number, the sum of the number of neutrons plus protons. For example, the element carbon has six protons. C-14 means “carbon with 8 neutrons” and C-12 means “carbon with 6 neutrons.”
THE BOHR ATOM In 1911, Niels Bohr proposed a “planetary” model of the atom. He theorized that electrons travel in nearly circular paths, called orbits, around the nucleus. Each electron orbit has a definite amount of energy. The farther away an electron is from the nucleus, the greater is its energy.
THE BOHR ATOM
2 | P a g e INTEGRATED SCIENCE MR. SURRETTE VAN NUYS HIGH SCHOOL
THE BOHR ATOM Bohr suggested that electrons “jump” between energy levels (orbits) in a quantum fashion. Quantum means the electrons never exist in-between orbits. When an atom absorbs or gives off energy like heat and light, the electron jumps to higher or lower orbits.
WAVELIKE PROPERTIES OF MATTER In 1925, Louis de Broglie proposed that particles behave like energy waves. According to de Broglie, all moving objects have a wavelength related to momentum. The letter h in the following equation is called Planck’s constant.
DE BROGLIE WAVELENGTHS
= h / mv Wavelength [m] = 6.626 x 10-34 J.s / (mass [kg])(velocity [m/s])
-31 Example 1. What is the de Broglie wavelength of an electron (me = 9.1 x 10 kg) traveling at 7.45 x 106 m/s? 1A. (1) = h / mv (2) = (6.626 x 10-34 J.s) / (9.1 x 10-31 kg)(7.45 x 106 m/s) (3) = 9.77 x 10-11 m
ELECTRON ORBITALS de Broglie’s wavelength explains the location of electron orbitals. For example, an electron traveling at 3 percent the speed of light (8.99 x 106 m/s) has a wavelength of 1 x 10-12 meter. This is about equal to the diameter of a hydrogen atom.
ELECTRON WAVES The circumference of the first electron orbital is equal to one wavelength of the electron wave. The second electron orbital has a circumference of two electron wavelengths, the third has three, and so forth. POSSIBLE VERSUS IMPOSSIBLE ELECTRON ORBITALS
3 | P a g e INTEGRATED SCIENCE MR. SURRETTE VAN NUYS HIGH SCHOOL
RADIOACTIVITY All elements with an atomic number greater than 82 (lead) are radioactive. These elements and a few others emit three types of radiation: alpha) beta) gamma)
ALPHA PARTICLES () An alpha particle is a combination of two protons and two neutrons (in other words, it is the nucleus of a helium atom). Alpha particles are relatively easy to stop because of their large size and double positive charge.
BETA PARTICLES () A beta particle is an electron ejected from a neutron inside the nucleus. This neutron becomes a proton once it loses the electron. A beta particle is faster than an alpha particle and carries a single negative charge.
GAMMA RAYS () Gamma rays are high-frequency electromagnetic radiation. Like visible light, gamma rays are pure energy. Because they have no mass nor electric charge, and because of their high energies, gamma rays penetrate through most materials.
RELATIVE STRENGTH OF RADIATION
ISOTOPES The number of neutrons in the nucleus of an atom varies. For example, the nucleus of every hydrogen atom contains one proton, but some hydrogen nuclei contain one or two neutrons in addition to the proton.
ISOTOPES OF HYDROGEN
4 | P a g e INTEGRATED SCIENCE MR. SURRETTE VAN NUYS HIGH SCHOOL
PROPERTIES OF NUCLEI 1. The atomic number Z equals the number of protons. 2. The neutron number N equals the number of neutrons. 3. The mass number A equals the number of nucleons (neutrons plus protons). The variables Z, N, and A are related by: A = Z + N
Example 1. If there are 128 neutrons in Pb-210, how many neutrons are in Pb-206? 1A. (1) A1 = Z + N1 (2) Z = A1 – N1 (3) Z = 210 – 128 (4) Z = 82 (5) A2 = Z + N2 (6) N2 = A2 – Z (7) N2 = 206 – 82 (8) N2 = 124
HALF-LIFE The rate of decay for a radioactive isotope is measured by its half-life. Half-life is the time it takes for half the original quantity of a radioactive element to decay. For example, radium-226 will be converted to other elements after 1620 years.
RADIUM HALF-LIFE
MEASURING HALF-LIFE The half-life of an element can be calculated by measuring the rate of decay of a known quantity. This is done using a radiation detector. In general, the shorter the half-life of a substance, the faster it disintegrates and more radioactivity is detected.
5 | P a g e INTEGRATED SCIENCE MR. SURRETTE VAN NUYS HIGH SCHOOL
RADIATION DETECTORS
NUCLEAR FORCES Within the nucleus of every atom there exists a balance between attractive nuclear forces and the repulsive electric forces between protons.
NUCLEAR FISSION In uranium, this nuclear balance is fragile. If a uranium nucleus stretches (see below), the electrical forces may push it into an elongated shape. If it stretches past a certain point, the repulsive electrical forces overwhelm the attractive nuclear forces and the nucleus splits. This is nuclear fission.
NUCLEAR DEFORMATION
CHAIN REACTION In a typical uranium chain reaction, one neutron starts the fission of uranium and releases 3 neutrons. These 3 neutrons strike 3 other uranium nuclei and produce 9 neutrons, and so on. This is called a chain reaction.
6 | P a g e INTEGRATED SCIENCE MR. SURRETTE VAN NUYS HIGH SCHOOL
START OF A CHAIN REACTION
CRITICAL MASS If enough nuclei are concentrated past the critical mass, an explosion of enormous magnitude can take place. The critical mass for uranium is about the size of a baseball.
CRITICAL MASS
BINDING ENERGY The total mass of the nucleus is always less than the sum of the masses of its individual particles. This “lost mass” is converted into the attractive nuclear force and is called the binding energy: E = mc2
(m = mass before reaction (mB) – mass after reaction (mA)
BINDING ENERGY CONVERSION One atomic mass unit converts into 931.5 million electron-volts of energy:
c2 = 931.5 MeV/amu
Example 1. What energy results when one hydrogen atom and two neutrons are combined to form a tritium atom? (hydrogen 1.007825 amu, neutron 1.009665 amu, tritium 3.016049 amu) 1A.
(1) m = mB - mA (2) mB = 1 H + 2 n (3) mB = 1.007825 amu + 2(1.009665 amu) (4) mB = 3.027155 amu 3 (5) mA = H
7 | P a g e INTEGRATED SCIENCE MR. SURRETTE VAN NUYS HIGH SCHOOL
1A. (continued…) (6) mA = 3.016049 amu (7) m = 3.027155 amu – 3.016049 amu (8) m = 0.011106 amu (9) E = mc2 (10) E = (m)c2 (11) E = (0.011106 amu)(1 amu / 931.5 MeV) (12) E = 10.34 MeV
NUCLEAR POWER PLANTS Modern nuclear power plants use nuclear fission. Research is currently underway to produce power plants that use nuclear fusion. A drawback to nuclear fission is the production of radioactive waste.
NUCLEAR FUSION Nuclear fusion is the combination of the nuclei of light atoms to form heavier nuclei.
THERMONUCLEAR FUSION Fusion brought about by high temperatures is called thermonuclear fusion. In the high temperatures of the Sun (20,000,000oC at its core), 657 million tons of hydrogen is fused into 653 tons of helium every second. The “missing” 4 million tons of mass becomes heat and light.
“COLD FUSION” The only known form of fusion is thermonuclear fusion. Scientists are searching for ways to create “cold fusion” devices that would operate at much lower temperatures.
8 | P a g e INTEGRATED SCIENCE